1,6-Dibromopyrene: properties, applications and safety
General Description
1,6-Dibromopyrene is a highly reactive and toxic aromatic compound with limited solubility in water, making it exist as a solid at room temperature. It is utilized in organic synthesis and materials science due to its ability to undergo various reactions, and it plays a crucial role in the creation of conjugated copolymers with exceptional iodine adsorption capabilities and metal ion capture potential. Additionally, 1,6-Dibromopyrene is essential in the controlled synthesis of parallel graphene nanoribbons with distinct edges and widths, holding significant potential for nanodevices. However, it requires careful handling and proper disposal to prevent health risks and environmental contamination. Strict adherence to safety regulations and guidelines is essential when working with this compound.

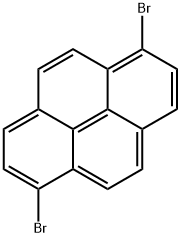
Figure 1. 1,6-Dibromopyrene
Properties
1,6-Dibromopyrene is a chemical compound with notable properties. It is a highly reactive and toxic aromatic compound, consisting of a pyrene backbone with two bromine substituents at the 1st and 6th positions. The physical properties of 1,6-Dibromopyrene include a high melting point and low solubility in water, indicating its propensity to exist as a solid at room temperature and its limited dispersibility in aqueous environments. Chemically, 1,6-Dibromopyrene is utilized in organic synthesis and materials science due to its ability to undergo various reactions, such as substitution and coupling reactions, making it valuable for the creation of complex organic molecules and functional materials. Its toxicity and reactivity also make it a subject of environmental concern, as exposure to 1,6-Dibromopyrene can have adverse effects on organisms and ecosystems. Overall, 1,6-Dibromopyrene's properties make it a significant compound in both chemical research and environmental impact studies due to its reactivity, toxicity, and limited solubility. 1
Applications
Creation of conjugated copolymers TPP1-3
1,6-Dibromopyrene, a compound synthesized through a palladium-catalyzed [3+2] cycloaddition polymerization with dialkynyl thiophene derivatives, plays a crucial role in the creation of three conjugated copolymers TPP1-3. These copolymers exhibit excellent yields and high purity, as verified by instrumental analyses. Notably, TPP1-3 demonstrate remarkable iodine adsorption capabilities, with a capacity of up to 3900 mg g-1, and follow a pseudo-second-order kinetic model for adsorption. Additionally, TPP3, one of the copolymers, displays efficient recyclability, maintaining its iodine uptake abilities even after multiple adsorption-desorption cycles. Moreover, these copolymers show promise in capturing nickel ions from water, with a maximum equilibrium adsorption capacity of 48.5 mg g-1. Therefore, the application of 1,6-Dibromopyrene in the synthesis of TPP1-3 copolymers contributes to the development of materials with significant potential for iodine adsorption and the capture of metal ions from aqueous environments. 2
Formation of parallel graphene nanoribbons
1,6-Dibromopyrene is utilized in the formation of extended patterns of parallel graphene nanoribbons with distinct edges and widths. This is achieved through surface-assisted Ullmann coupling polymerization and dehydrogenation processes, as evidenced by scanning tunneling microscopy, X-ray spectroscopy, and density functional theory calculations. The resulting nanostructures hold significant potential for applications in nanodevices. Additionally, the unique properties of halogenated pyrene derivatives, particularly on Ag(110), make them especially suitable for nanoribbon formation. These findings suggest that the shape and dimensions of nanoribbons, along with their electronic properties, can be controlled by selecting appropriately designed molecular precursors. Therefore, 1,6-Dibromopyrene plays a crucial role in the controlled synthesis and potential customization of graphene nanoribbons for various technological applications. 3
Safety
1,6-Dibromopyrene is a chemical compound that requires careful handling due to potential safety concerns. This substance poses health risks if inhaled, ingested, or comes into contact with the skin or eyes. It may cause irritation, sensitization, or other adverse effects on human health. Additionally, 1,6-Dibromopyrene should be stored and disposed of properly to prevent environmental contamination. When working with this compound, appropriate personal protective equipment and safety measures must be used to minimize exposure. Furthermore, it is crucial to adhere to established regulations and guidelines for the safe handling, transportation, and use of 1,6-Dibromopyrene to ensure overall safety. 4
Reference
1. PubChem. COMPOUND SUMMARY: 1,6-Dibromopyrene. National Library of Medicine, PubChem CID: 176470.
2. Shetty S, Baig N, Wahed SA, Hassan A, Das N, Alameddine B. Iodine and Nickel Ions Adsorption by Conjugated Copolymers Bearing Repeating Units of Dicyclopentapyrenyl and Various Thiophene Derivatives. Polymers (Basel). 2023 Oct 19;15(20):4153.
3. Smerieri M, Píš I, Ferrighi L, Nappini S, Lusuan A, Di Valentin C, Vaghi L, Papagni A, Cattelan M, Agnoli S, Magnano E, Bondino F, Savio L. Synthesis of graphene nanoribbons with a defined mixed edge-site sequence by surface assisted polymerization of (1,6)-dibromopyrene on Ag(110). Nanoscale. 2016 Oct 20;8(41):17843-17853.
4. 1,6-Dibromopyrene. European Chemicals Agency, EC / List no. 881-564-2.
You may like
Related articles And Qustion
See also
Lastest Price from 1,6-Dibromopyrene manufacturers

US $30.00-10.00/KG2025-04-15
- CAS:
- 27973-29-1
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $0.00-0.00/kg2025-04-04
- CAS:
- 27973-29-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton