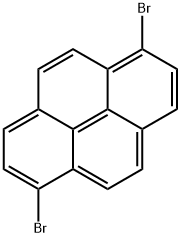
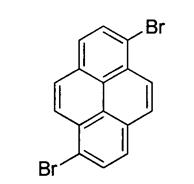
Preparation Method for 1,6-Dibromopyrene
1, 6-dibromopyrene is a kind of pyrene chemical. It is majorly used as organic intermediate, OLED (organic light-emitting diodes) materials and photoelectric material. It, together with its 1, 8- isomer, can be produced by the double bromination of pyrene with bromine. It can be applied to the synthesis of bipyridine and terpyridine based ruthenium metallosynthons. Here, we introduce two preparation methods for this compound [1].
A classical method reported in 1972
1,6-Dibromopyrene can be synthesized according to the method reported in 1972 by J. Grimshaw and J. Trocha-Grimshaw [2]. To a stirred solution of pyrene (12.2 g, 60 mmol) in CCl4 (300 ml) under N2 at room temperature was added dropwise a CCl4 solution (50 ml) of bromine (6.32 ml, 0.126 mol) over a period of 4 h. After the mixture had been stirred for 48 h, the precipitate was collected by filtration, washed with methanol and recrystallized from toluene to afford dibromide 6 (8.3 g, 38.4%), mp 210–211 8C; dH(250 MHz; [2H6]DMSO; 130 8C) 8.25 (2H, d, J 5.15, 3- and 8-H), 8.33 (2H, d, J 5.80, 4- and 9-H), 8.36 (2H, d, J 5.15, 2- and 7-H) and 8.42 (2H, d, J 5.80, 5- and 10-H) (Found: C, 53.37; H, 2.24. C16H8Br2 requires C, 52.45; H, 2.24%)[3].
An improved method in 2011(CN103102244A)
Another method to prepare 1,6-dibromopyrene is recorded in a patent in 2011 with the publication number of CN103102244A. The invention discloses a preparation method for 1,6-dibromopyrene. The preparation method is characterized by comprising the following steps: dissolving pyrene in an organic solvent; adding dibromohydantoin for a reaction; carrying out filtering; and subjecting an obtained solid to recrystallization so as to obtain 1,6-dibromopyrene.
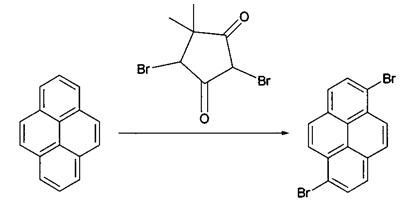
The preparation method provided by the invention has the advantages of easiness, mild reaction conditions, high yield and convenience in industrial production. In a bromination reaction in the method, dibromohydantoin is employed, and bromination of liquid bromine is avoided, wherein liquid bromine has strong toxicity and corrosivity, and at a normal temperature, liquid bromine can volatilize strongly irritant smog to irritate mucosas of eyes and respiratory tracts, which incurs tearing and coughing of people, and can burn the skin and cause a sharp pain, which is hard to heal. Bromine has active properties, is a strong oxidizing agent, violently reacts and is difficult to control. When hydrobromic acid and hydrogen peroxide are used as brominating agents, considerable strong acid waste water is produced after a reaction is finished, which causes severe pollution [4].
Reference
[1]https://www.chemicalbook.com/ChemicalProductProperty_EN_CB8947912.htm
[2] Grimshaw, James, and J. Trocha-Grimshaw. "Characterisation of 1, 6-and 1, 8-dibromopyrenes." Journal of the Chemical Society, Perkin Transactions 1 (1972): 1622-1623.
[3] Suenaga, Hikaru, et al. "Pyrenylboronic acids as a novel entry for photochemical DNA cleavage: diradical-forming pyrene-1, 6-diyldiboronic acid mimics the cleavage mechanism of enediyne antitumor antibiotics." Journal of the Chemical Society, Perkin Transactions 1 7 (1998): 1263-1268.
[4] https://patents.google.com/patent/CN103102244A/en
You may like
Related articles And Qustion
Lastest Price from 1,6-Dibromopyrene manufacturers

US $30.00-10.00/KG2025-04-15
- CAS:
- 27973-29-1
- Min. Order:
- 50KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $0.00-0.00/kg2025-04-04
- CAS:
- 27973-29-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton