1,1-Difluoroethane: Properties, Production process and Uses
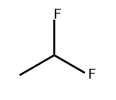
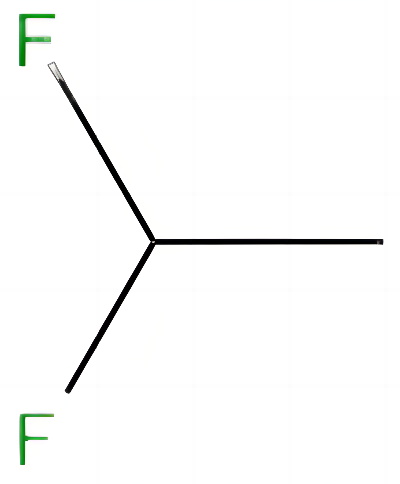
1,1-Difluoroethane, also known as R-152a, HFC-152a, is a derivative of acetylene and hydrogen fluoride. Molecular formula: C2H4F2, molecular weight: 66.0500. The chemical structure is:
Properties of 1,1-difluoroethane
1,1-Difluoroethane is a colorless gas at room temperature. It can easily be liquefied. Its melting point and boiling point are −117°C and −24.7°C, respectively. The enthalpy of vaporization is 22.7 kJ.mol–1. It is insoluble in water. The explosion limit in air is from 3.7% to 18%.
Processes for manufacture of 1,1-difluoroethane
According to different raw materials, the processes for manufacture of 1,1-difluoroethane include two routes: fluorination of acetylene and fluorination of vinyl chloride.
Fluorination of acetylene
Acetylene undergoes a fluorination reaction with anhydrous hydrogen fluoride to produce 1,1-difluoroethane:
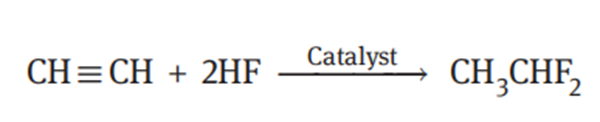
The synthesis of 1,1-difluoroethane from acetylene and HF has the advantages including simple process, easily available raw materials and low cost. The reaction can be
carried out either in liquid phase or in gas phase. In the liquid phase, fluorosulfonic acid is used as the catalyst, the reaction is carried out at a temperature of
30°C under a pressure of 0.03–0.3 MPa. In the gas phase, AlF3 is generally used as a
catalyst, and the reaction is carried out at 220–280°C under atmospheric pressure.
The gas-phase process has the advantages including higher catalytic activity and
conversion (>98%). Moreover, the gas-phase process does not have problems such as
high corrosion of the catalyst and difficulty in the treatment of the wastes as that in
the liquid-phase process. However, the surface of the catalyst is easily coked during
the reaction, resulting in catalyst deactivation. Addition of Mn element to the catalyst improved the reaction selectivity slightly. The coking of the catalyst can be suppressed to some extent by addition of Bi element to the catalyst.
Fluorination of vinyl chloride
The raw materials for production of 1,1-difluoroethane by the fluorination of vinyl chloride are vinyl chloride and anhydrous hydrogen fluoride. The reaction process includes addition and substitution. Vinyl chloride undergoes an addition reaction with HF to generate CH3CHClF, and then the resultant CH3CHClF reacts with HF, leading to fluorine atom in substitution for chlorine atom and generation of 1,1-difluoroethane:
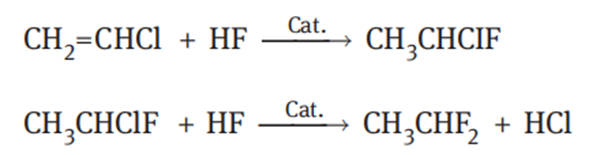
The catalyst is generally selected from the halides of titanium, vanadium, tantalum, molybdenum, tungsten, tin and stibium. It is recommended to use SnCl4 as a catalyst. During the operation, hydrogen fluoride and vinyl chloride are continuously introduced into the reaction vessel, and the reaction mixture is continuously discharge from the reactor. The residence time is generally 0.1–5 h. Although industrial production of 1,1-difluoroethane by fluorination of vinyl chloride has accounted for a large proportion, the process still has the following problems: First, the service life of the catalyst is short, the production capacity is small, and the unit consumption is high. It is difficult to increase the capacity of a single kettle. Second, severe fouling of carbonaceous solids on the reactor wall occurs because of a large amount of high boiling by-products such as polymers and oily substances. Third, the conversion of vinyl chloride is not high enough, and the unreacted vinyl chloride forms an azeotrope with 1,1-difluoroethane, which makes it difficult to purify 1,1-difluoroethane and thus limits the application of 1,1-difluoroethane in many fields.
Some efforts have been made to solve the above problems. Huang et al. optimized and improved the fluorination process, so that the efficiency of the catalyst (reaction material mass/catalyst mass) increased from 60–80 g.g–1 to 130–150 g.g–1; vinyl chloride feed rate from 0.1–0.18 h−1 was increased to 0.28–0.32 h−1, the productivity of a single kettle was greatly improved, and the carbonaceous solid matter on the reactor wall and the high-boiling substances such as polymers and oily substances formed by the reaction were greatly reduced. The product quality has been significantly improved. Chen proposed a two-step method to produce 1,1-difluoroethane, which improved the conversion and selectivity of the reaction. The conversion of vinyl chloride was complete, so that there is no need to separate or use photochlorination and other methods to remove vinyl chloride.
Uses of 1,1-difluoroethane
1. For refrigerant
The production and use of chlorofluorocarbons have been limited because of the Montreal Protocol. HFC-152a has an ozone depletion potential (ODP) of zero, which is receiving increasing attention as a substitute for chlorofluorocarbons. For example, on 12 June 2008, the US Environmental Protection Agency announced that HFC-152a was included in the list of alternative refrigerants for automotive air conditioning.
HFC-152a can be used alone as a refrigerant or mixed with other refrigerants. For
example, a mixture of HFC-152a and F-12 in a mass ratio of 26.2:73.8 has greater refrigeration capacity. The refrigerant mixture of HFC-152a and HFC-134a has better environmental performance and thermodynamical performance than HFC-134a alone,
which can reduce energy consumption and cost.
2. For foaming agent
In view of the need for environmental protection, chlorofluorocarbons, which have
traditionally been used for foaming agents, will be eliminated. Hydrocarbon foaming
agents are specified as photochemically reducing volatile organic compounds (VOCs),
and their use is also severely restricted. Therefore, 1,1-difluoroethane is often used as
a foaming agent in place of trichlorofluoromethane for foaming of plastic products.
3. For the manufacture of other products
1,1-Difluoroethane is also used as an intermediate in organic synthesis for the synthesis of vinylidene fluoride, an important monomer for fluororubbers and fluoroplastics. For example, 1,1-difluoroethane is used for production of PVDF resin, and
typical manufacturers include Pennwalt Company (United States). In China, Shanghai Institute of Organic Materials, Chenguang Chemical Research Institute and Zhejiang Research Institute of Chemical Industry are also producing PVDF resin from
1,1-difluoroethane.
4. For heat pump working fluid
1,1-Difluoroethane can be used as a working fluid for heat pump. The two materials, HFC-152a and 1,1,1,3,3,3-hexafluoropropane, are physically mixed at a room temperature to form a corresponding mixed working medium, which is suitable for medium and high temperature heat pump units. The mixed working medium does not destroy the ozone layer, meeting environmental protection requirements. It has suitable thermal parameters and excellent cycle performance.