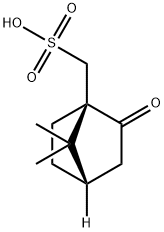
(1R)-(-)-10-Camphorsulfonic acid: Uses and Mechanism
Uses of (1R)-(-)-10-Camphorsulfonic acid
(1R)-(-)-10-Camphorsulfonic acid is a non-alkyl acid containing an oxidising group that may be involved in hydrogen bonding and is mainly used to separate optically active isomers.
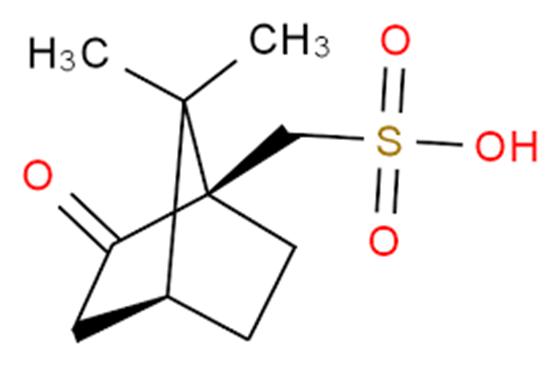
(1R)-(-)-10-Camphorsulfonic acid is a derivative of camphorsulfonic acid (CSA), which is often used as a catalyst in organic synthesis reactions. (S)-CSA was used in the resolution of 2-piperidineethanol, a chiral starting material for the asymmetric synthesis of (-)-isooctanodine, a spermine alkaloid with a 22-membered lactam. D-CSA, a chiral Brønsted acid, is a practical and efficient catalyst for the enantioselective Michael Friedel-Crafts reaction of indoles with aromatic alkenones, giving the corresponding β-indolones in excellent yields and moderate enantioselectivity. (1R)-(-)-10-Camphorsulfonic acid is a derivative of camphorsulfonic acid (CSA), which is often used as a catalyst in organic synthesis reactions.
(S)-CSA was used in the resolution of 2-piperidineethanol, a chiral starting material for the asymmetric synthesis of (-)-isooctanodine, a spermine alkaloid with a 22-membered lactam. The enantioselective Henry (nitroaldol) reaction between nitromethane and aromatic aldehydes was successfully catalysed by a chiral iminopyridine copper complex prepared by CSA. (S)-CSA was used as a chiral acid to induce optical activity of polyaniline emeraldine salt (PAn-HA).
(1R)-(-)-10-Camphorsulfonic acid is also used to prepare polyaniline (PANI) films. Polyaniline (PANI) films made from formic acid cast with CSA as the dopant reduce the film resistance and remain stable for at least 4 months.
Mechanism
(1R)-(-)-10-Camphorsulfonic acid isolates enantiomers of amino alcohols, mainly beta-blockers (derivatives of 1-aryl-2-amino alcohols). The aromatic portion of these solutes is attached to the side chain. It was found that selectivity depends on the distance between the hydroxyl and amine groups, implying that both structural features play an important role in chiral recognition.
An example of the interaction between (+)-10-camphorsulfonic acid and β-blockers was used. Multiple chiral separations were carried out using (+)-10-camphorsulfonic acid in dichloromethane:1-pentanol (199:1) as mobile phase and DIOL stationary phase. When DIOL functionalized packing and sulfonic acid were used as mobile phases, it was crucial to select solvents with low content of polar components as mobile phases to facilitate the formation of ion pairs. The use of Br-3-(+)-10-camphorsulfonic acid for the separation of β-blockers in systems using DIOL stationary phases was not successful. For these solutes and in this particular system, it was believed that the Br group sterically impeded the three-point contact between the selector and the solute. Moving the sulfonic acid group from the oxygen group to the 8th position (Br-3-(+)-8-camphorsulfonic acid) restored stereoselectivity to some extent.
References:
[1] ISAO SASAKI Mira J Ji?í Janata. Stabilization of electronic properties of (1R)-(?)-10-camphorsulfonic acid doped polyaniline by UV irradiation[J]. Polymer Degradation and Stability, 2007, 92 7: 1161-1420. DOI:10.1016/j.polymdegradstab.2007.02.021.You may like
Related articles And Qustion
See also
Lastest Price from (1R)-(-)-10-Camphorsulfonic acid manufacturers

US $0.00-0.00/kg2025-10-09
- CAS:
- 35963-20-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $3.00/kg2025-04-21
- CAS:
- 35963-20-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000