(1R)-(-)-10-Camphorsulfonic Acid: Role in Biomaterials for Neural Applications and its Preparation Method
General Description
(1R)-(-)-10-Camphorsulfonic acid is a versatile compound crucial for material science and biomedical applications, particularly in the development of electroconductive polymers like poly(aniline). (1R)-(-)-10-Camphorsulfonic acid enhances polymer conductivity, facilitating effective neural cell cultivation and promoting differentiation through electrical stimulation. Its preparation via bipolar membrane electrodialysis ensures high purity and efficiency, converting ammonium (1R)-(-)-10-camphorsulfonate with over 98% yield. The method is environmentally sustainable, eliminating the need for chemical reagents and reducing waste, thereby maximizing (1R)-(-)-10-Camphorsulfonic acid's potential in innovative therapeutic strategies for neural regeneration and disease modeling in biomedical research.

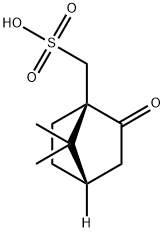
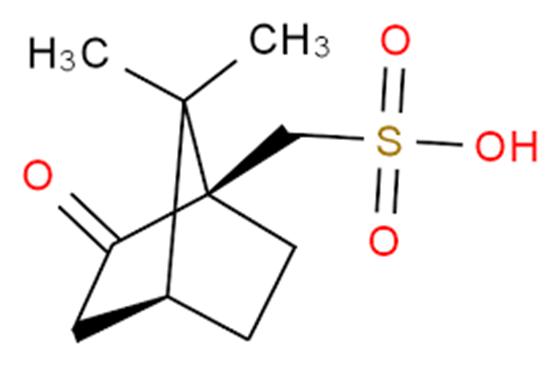
Figure 1. (1R)-(-)-10-Camphorsulfonic acid
Role in Biomaterials for Neural Applications
(1R)-(-)-10-Camphorsulfonic acid is a versatile compound widely used in various fields, particularly in the realm of material science and biomedical applications. As a chiral auxiliary, (1R)-(-)-10-Camphorsulfonic acid plays a crucial role in asymmetric synthesis, helping to produce enantiomerically pure compounds that are essential in pharmaceuticals. Its ability to form stable salts with amines enhances the solubility and accessibility of certain compounds, making it an invaluable tool in both organic chemistry and medicinal chemistry. The application of (1R)-(-)-10-Camphorsulfonic acid is especially pronounced in the development of electroconductive polymers, where it acts as a dopant to improve the electrical properties of materials. 1
Application in Polymer Science
In the context of polymer science, (1R)-(-)-10-Camphorsulfonic acid serves as a protonating agent for the synthesis of electroconductive polymers such as poly(aniline). This polymer demonstrates significant promise for neural cell cultivation, particularly when combined with electrical stimulation techniques. The doping effect of (1R)-(-)-10-Camphorsulfonic acid enhances the conductivity of poly(aniline), enabling efficient charge transfer that is critical for stimulating neural tissues. These properties facilitate the design of scaffolds and platforms where (1R)-(-)-10-Camphorsulfonic acid-modified poly(aniline) can direct electrical fields to neural cells, promoting their differentiation and maturation into functional neurons. This is particularly important for applications such as nerve regeneration and the development of drug screening platforms that rely on neural cell behavior. 1
Supporting Neural Regeneration
As research advances, the significance of (1R)-(-)-10-Camphorsulfonic acid in promoting neural cell differentiation through electrical stimulation becomes increasingly clear. The combination of this compound with advanced biomaterials paves the way for innovative therapeutic strategies in treating neurological disorders. By optimizing the properties of electroconductive polymers using (1R)-(-)-10-Camphorsulfonic acid, researchers can create more effective platforms that mimic the natural extracellular matrix, significantly enhancing the performance of neural cells in culture. This leads to the potential for successful neural regeneration and the creation of more effective strategies for disease modeling, thereby broadening the scope of (1R)-(-)-10-Camphorsulfonic acid within biomedical research and applications. 1
Preparation Method
The preparation method of (1R)-(-)-10-camphorsulfonic acid employs bipolar membrane electrodialysis as an efficient technique. In this process, an aqueous solution of ammonium (1R)-(-)-10-camphorsulfonate is introduced into the salt chamber of the bipolar membrane stack. Simultaneously, deionized water is fed into both the acid and alkali chambers. Additionally, strong electrolyte solutions such as sodium sulfate or sodium nitrate are added to the anode and cathode chambers. By applying a direct current across the chambers at a density of 10-100 mA/cm², the transformation reaction occurs, resulting in (1R)-(-)-10-camphorsulfonic acid. This method guarantees a conversion rate exceeding 98% for ammonium (1R)-(-)-10-camphorsulfonate, with the product exhibiting a purity level of more than 99%. 2
Advantages of the Electrodialysis Method
This innovative preparation method of (1R)-(-)-10-camphorsulfonic acid not only enhances product purity but also simplifies operations by eliminating the need for chemical reagents. During the electrodialysis process, the bipolar membrane efficiently dissociates water to generate protons (H+) and hydroxide ions (OH-), facilitating the direct conversion of ammonium (1R)-(-)-10-camphorsulfonate to (1R)-(-)-10-camphorsulfonic acid along with the production of ammonia. The configuration of the bipolar membrane stack allows for optimal flow rates, preventing concentration polarization and maximizing the effectiveness of the reaction. Additionally, this method offers significant environmental benefits, as it avoids the use of ion exchange resins and reduces the generation of chemical waste, presenting a green and economically sustainable approach for producing (1R)-(-)-10-camphorsulfonic acid in industrial applications. 2
References:
[1] FáBIO F F GARRUDO. Designing Electrical Stimulation Platforms for Neural Cell Cultivation Using Poly(aniline): Camphorsulfonic Acid.[J]. Polymers, 2023, 15 12. DOI:10.3390/polym15122674.You may like
Related articles And Qustion
Lastest Price from (1R)-(-)-10-Camphorsulfonic acid manufacturers

US $0.00-0.00/kg2025-10-09
- CAS:
- 35963-20-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $3.00/kg2025-04-21
- CAS:
- 35963-20-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10000