Factory Provide High Quality Antiretroviral Efavirenz Powder CAS 154598-52-4 Efavirenz

Introduction
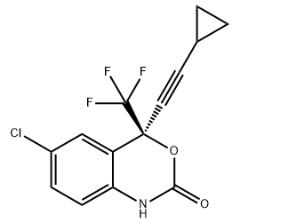
Product Name : Efavirenz
Other name: (S)-6-Chloro-4-(cyclopropylethynyl)-1,4-dihydro-4-(trifluoromethyl) 2H-3,1-benzoxazin-2-one;(4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluoromethyl)-1H-3,1-benzoxazin-2-one;
CAS No.: 154598-52-4
Molecular Formula: C14H9ClF3NO2
Molecular Weight: 315.67
Specification:99%
Appearance: White powder or crystal
Melting Point :139-141°C (rough estimate)
Boiling Point :340.6±42.0 °C(Predicted)
Density: 1.53±0.1 g/cm3(Predicted)
Storage :Keep in dark place,Sealed in dry,Store in freezer, under -20°C
Efavirenz D5 (Sustiva)84 is also mandated for use with at leasttwo other antiretroviral agents. The compound is morethan 99% protein bound, and CSF concentrations exceedthe free fraction in the serum. Metabolism occurs in theliver. The half-life of a single dose of Efavirenz D5 is 52 to 76hours, and 40 to 55 after multiple doses (the drug inducesits own metabolism). Peak concentration is achieved in 3to 8 hours. Elimination is 14% to 34% in urine (as metabolites)and 16% to 41% in feces (primarily as Efavirenz D5).The oral dosage form is supplied as a capsule.
Function of Efavirenz
Efavirenz is one of the most commonly prescribed antiretroviral medications in the world. Several large randomized, controlled trials[RCTs] have demonstrated the superior ability of efavirenz to suppress the HIV virus when used as part of a 3-drug cocktail[2]. The introduction of Atripla in 2006 combined efavirenz and two other agents into a single pill, once-daily formulation. This formulation was more convenient and better tolerated than alternatives, and thus resulted in improved adherence to antiretroviral medications[3]. However, neuropsychiatric side effects associated with efavirenz have been reported to occur in 40-50% of patients and represent one of the limiting factors of this regime
Application of Efavirenz
For use in combination treatment of HIV infection (AIDS)

 China
China