| Product Name | Cuprous oxide |
| CAS No | 1317-39-1 |
| EC-No | 215-270-7 |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 50000KG/month |
| Release date | 2025/03/21 |
| CAS: | 1317-39-1 |
| MF: | Cu2O |
| MW: | 143.09 |
| EINECS: | 215-270-7 |
| Product Categories: | metal oxide;paint;Inorganics;Dye;Chemical Synthesis;Chemistry;29: Cu;Catalysis and Inorganic Chemistry;Chemical Synthesis;CopperMaterials Science;Nanomaterials;Nanoparticles: Oxides, Nitrides, and Other CeramicsNanomaterials;Nanopowders and Nanoparticle Dispersions;1317-39-1 |
| Mol File: | 1317-39-1.mol |
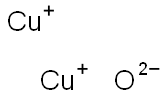 | |
| Cuprous oxide Chemical Properties |
| Melting point | 1232 °C |
| Boiling point | 1800 °C |
| density | 6 g/mL at 25 °C (lit.) |
| refractive index | 2.705 |
| Fp | 1800°C |
| storage temp. | Inert atmosphere,Room Temperature |
| form | powder |
| color | red |
| Specific Gravity | 6. |
| Water Solubility | practically insoluble |
| Sensitive | Air & Moisture Sensitive |
| Merck | 14,2664 |
| Exposure limits | ACGIH: TWA 1 mg/m3 NIOSH: IDLH 100 mg/m3; TWA 1 mg/m3 |
| Stability: | Stable. May be air or light sensitive. |
| CAS DataBase Reference | 1317-39-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Copper(i) oxide(1317-39-1) |
| EPA Substance Registry System | Cuprous oxide (1317-39-1) |
| Uses | 1. It can be used for coating of ship bottom coating, pesticide germicide, and glaze and used for copper oxide rectifier, photocell, electroplating and copper salt production. 2. It can be used in the manufacture of antifouling paint (used to kill low-level marine animals), pesticides, and various copper salts, analytical reagents and red glass. It can also be used in the rectifier plating in the electrical industry. It can also be used as the coloring agent for ceramic and enamel. 3. It can be used as analytical reagents and germicide. 4. It can be used as the reducing agent for determination of nitrogen content in azo compounds, red glaze, electroplating, germicide, red glass, ship bottom paint, plant seeds sterilization and catalyst. |
| Production method | Dry copper powder, after the removal of impurities, is mixed with copper oxide and sent into the calcination furnace for being heated to 800~900 ℃ to be calcined into cuprous oxide. After taking out, use a magnet to suck the mechanical impurities and then smashed to 325 meshes, making cuprous oxide products. If taking copper sulfate as raw materials, we should first use iron to reduce the copper contained in the copper sulfate out. The subsequent reaction steps are the same as that of the method using copper powder as raw material. Its reaction process is: Cu + CuO→Cu2O Glucose reduction method will be copper sulfate solution mixed with glucose and sodium hydroxide solution was added to the reaction, the formation of cuprous oxide, filtered, rinsed, dried crushed system in the cuprous oxide products. CuSO4 + 2NaOH → Na2SO4 + Cu (OH) 2 ↓ 2Cu (OH) 2 + CH2OH (CHOH) 4CHO → Cu2O ↓ + 2H2O + CH20H (CHOH) 4COOH Electrolytic method: in the iron-lined electrolytic cell with PVC lining, copper plate is used as anode and copper plate as cathode, potassium chromate is used as additive and salt solution as electrolyte, which contains 290 to 310 g/L of sodium chloride, 0.3 to 0.5 g/L of potassium chromate. Electrolysis is carried out under the condition that the temperature is 70-90 °C, Ph is 8 to 12 and the current density is 1500 A/m2, we can produce cuprous oxide with the precipitate being separated, rinsed, filtered and dried to obtain cuprous oxide. Its reaction is: Cathode reaction: 2H ++ 2e → H2O ↑ Anodic reaction: Cu-2e → Cu + 2Cu ++ 2C1-→ Cu2C12 Cu2C12 + 2NaOH → Cu2O ↓ + H2O + 2NaCl |
| Hazards & Safety Information | Category : Pesticides Toxicity grading: highly toxic Acute Toxicity: Oral-rat LD50 470 mg/kg; celiac-mouse LD50: 380 mg/kg Flammability and Hazardous properties: Non-combustible with fire producing toxic copper-containing fumes Storage and transportation characteristics : Treasury should be of low temperature, good ventilation and being drying; store it separately from food raw materials. Fire extinguishing agent : water, carbon dioxide, dry powder, sand Professional Standard : TWA 0.1 mg Cu/m3; STEL 0.2 mg Cu/m3 |
| Chemical Properties | Copper(I) oxide, Cu2O, occurs in nature as the red or reddish brown mineral cuprite in a cubic or octahedral crystalline morphology. Synthetic materials variously appear as yellow (somewhat pyrophoric), orange, red, or purple powders, depending on particle size. Copper(I) oxide is stable in dry air, but slowly oxidizes to copper(II) oxide, [1317-38-0], in moist air. It is virtually insoluble in water but dissolves in complexing acids such as HCl and in aqueous ammonia to form copper(I) complexes. In dilute sulfuric and nitric acids, disproportionation to the soluble copper(II) salts and copper powder obtains. |
| Uses | Fungicide; antiseptic for fishnets; in antifouling paints for marine use; in photoelectric cells; as red pigment for glass, ceramic glazes; in brazing pastes; in rectifiers; as catalyst. |
| Uses | Copper (I) oxide is used as red pigment for glass; in antifouling paints; fungicide. |
| Uses | Red pigment for glass and ceramic glazes, and as a catalystCopper(I) oxide is used in antifouling, marine paint, brazing paste, antifungal treatment for plant seeds. Used as a hydrogen scavenger in copper brass and bronze and high grade lubricants. Finds uses in glass and ceramic pigments, ferrite magnets, catalyst in organic synthesis, dyes and dye intermediates and as catalysts. |
| Definition | For the native ore see cuprite. |
| Definition | An insoluble red powder prepared by the heating of copper with copper(II) oxide or the reduction of an alkaline solution of copper(II) sulfate. Copper(I) oxide is easily reduced by hydrogen when heated; it is oxidized to copper(II) oxide when heated in air. It is used in the glass industry. Copper( I) oxide undergoes disproportionation in acid solutions, producing copper(II) ions and copper. The oxide will dissolve in concentrated hydrochloric acid due to the formation of the complex ion [CuCl2]-. Copper(I) oxide is a covalent solid. |
| Preparation | Copper(I) oxide decomposes above 1800°C but can be prepared by heating copper metal in air above 1030°C. To prevent further oxidation, the material must be cooled in an inert atmosphere. Lower temperatures may be used to produce the oxide if the material is blended with a reducing agent such as carbon and heated to 750°C in an inert atmosphere. The product must be stabilized with isophthalic acid or pine oil (Ayers, 1953). A particularly stable copper(I) oxide can be prepared by blending copper powder and cupric oxide in stoichiometric amounts and heating to 800-900°C. Lower temperatures can be used if ammonia or certain ammonium salts are added to the blend (Day, 1969; Drapeau and Johnson, 1956; Drapeau and Johnson, 1959). |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
-








