| Physical and Chemical Properties |
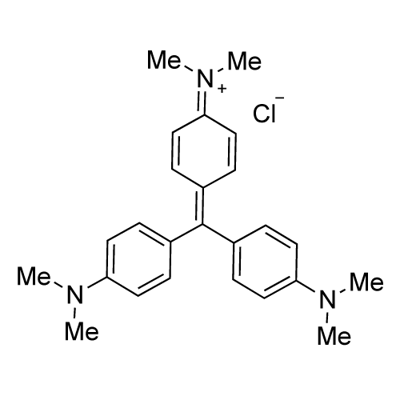
It is also known as "purple", "methyl violet", "gentian violet." It belongs to triphenylmethane type alkaline dyes. Scientific name:"hexamethyl chloride rose aniline." It appears as dark green powder with metallic luster. It is soluble in water, alcohol and chloroform, but insoluble in ether. Its solubility in water is 1.68% and its solubility in 95% ethanol is 13.87%. However, the solubility of its iodide in two solvents is only 0.035% and 1.78% respectively. Both its aqueous solution and alcohol solution are purple. The structural formula of its chloride is: 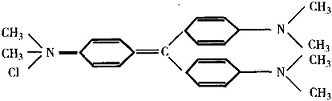 |
| Application |
It can be used as the dyes of silk, paper and acrylic as well as biological stains. It can be used for manufacturing paints and printing inks. It can also used as an acid-alkaline indicator with coloring range being from pH 0.5 (green) to 2.0 (blue), and developing reagent for colorimetric assays.
It can form a colored chelate with thallium in the hydrobromic acid medium, thus it can be used as thallium sensitivity reagent. It can also be used for the determination of other metal ions such as zinc, antimony, titanium, cadmium, tungsten, gold, mercury and so on. It can also be used as biological stains and non-aqueous titration acid-base indicator. In addition, it can also be used as antiseptic for inhibiting Staphylococcus aureus, Streptococcus and some fungi. Its 1% aqueous solution is commonly known as "purple syrup" for the prevention and treatment of skin and mucous membrane infections. Its enteric-coated tablets can be used as orally anti-pinworm medicine. |
| Chemical Properties |
dark green powder or crystals |
| Description |
Anti-infective (topical). Has been used as anthelmintic (Nematodes), as indicator for copper salts. |
| Uses |
As dye for wood, silk, paper; in inks; as biological stain. |
| Preparation |
It is manufactured through using N, N-dimethylaniline as raw materials, followed by condensation, addition, chlorination and other reactions. Alternatively, it can be synthesized through the reaction between Michler ketone and N, N-dimethylaniline reaction in the presence of phosphorus oxychloride, followed by azeotropic reaction with hydrochloric acid.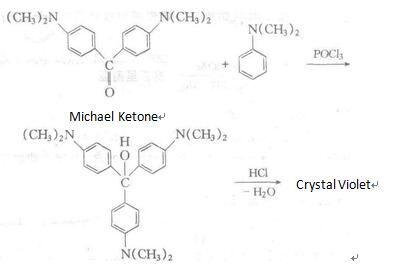 Recrystallization in hot water will generate compound containing nine crystal water molecules. Recrystallization in hot water will generate compound containing nine crystal water molecules. |
| General Description |
It belongs to triamino-triphenylmethane synthetic dyes; alkaline. It is an important dye used in bacterial Gram stain. The mixed reagent of crystal violet chloride and iodine is known as gentian violet. |
| Air & Water Reactions |
Insoluble in water. |
| Description |
Crystal Violet is light sensitive. May react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release gaseous hydrogen. |
| Fire Hazard |
Flash point data for Crystal Violet are not available, however, Crystal Violet is probably combustible. |
| Toxicity |
LD50 orally in mice, rats: 1.2, 1.0 g/kg (Hodge) |

 China
China



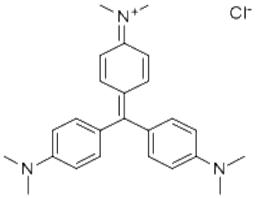


 Recrystallization in hot water will generate compound containing nine crystal water molecules.
Recrystallization in hot water will generate compound containing nine crystal water molecules.