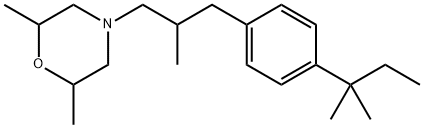
Amorolfine is available for topical use in 0.25% cream and 5% nail lacquer formulation for the treatment of onychomycosis in European countries and Japan. However, amorolfine is not approved in the USA for such indications. The major advantage of this new antimycotic drug presumably lies in its potential for use as 5% nail lacquer in the treatment of onychomycosis caused by dermatophytes and Candida spp.
Topical application of amorolfine (5% nail lacquer and 0.25% cream) is generally well tolerated, with up to 5% of patients reporting minor symptoms. In patients using the nail lacquer, these events included burning, itching, redness, and local pain, which were toleratable and confined to the application site. Adverse effects of scaling, weeping, blistering, and edema have also been described for the cream formulation.