The synthesis of Mycophenolic acid (Canonica L. Et al., Tetrahedron Letters,
1971, N 28, p.2691-2692)
By condensation of sodium diethylmalonate and 3-methylpent-3-en-2-on in
ethanol was obtained 2,3-dimethyl-4,6-dioxocyclohexanecarboxilic acid ethyl
ester, which was aromatised to 4,6-dihydroxy-2,3-dimethylbenzoic acid ethyl
ester (melting point 115-116°C). By treatment with diazomethane or with
CH3I and K2CO3 this compound was transformed into 2,4-dimethoxy-5,6-
dimethylbenzoic acid ethyl ester (melting point 62-63°C). The hydrolysis of
the ester group furnished the 2,4-dimethoxy-5,6-dimethylbenzoic acid
(melting point 208-210°C), which was converted into the amide: carbamic
acid 3-methoxy-4,5,6-trimethylphenyl ester (melting point 225-229°C).
Treatment of the amide with t-butylhypochlorite in methylene dichloride
yielded the corresponding N-chloroamide which was photolysed to the
intermediate iminolactone and was immediately hydrolized to 5,7-dimethoxy-
4-methyl-3H-isobenzofuran-1-one.
This compound with hydriodic acid in acetic acid in the presence of red
phosphorous at reflux yielded 5,7-dihydroxy-4-methyl-3H-isobenzofuran-1-
one. Condensation of 6-bromo-4-methylhex-4-enoic acid methyl ester and
5,7-dihydroxy-4-methyl-3H-isobenzofuran-1-one with silver oxide in dioxane
at room temperature yielded 6-(4,6-dihydroxy-7-methyl-3-oxo-1,3-dihydro�isobenzofuran-5-yl)-4-methylhex-4-enoic acid methyl ester (36% yield). At
last, monomethylation with diazomethane yield 6-(4-hydroxy-6-methoxy-7-
methyl-3-oxo-1,3-dihydro-isobenzofuran-5-yl)-4-methylhex-4-enoic acid methyl ester, which was hydrolysed with aqueous sodium hydroxide to 6-(4-
hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihydro-isobenzofuran-5-yl)-4-
methylhex-4-enoic acid (Mycophenolic acid).
Mycophenolic acid may be obtained by the fermentation broth of Pennicillium
brevicompactum. The synthesis of Mycophenolate mofetil (Patent U.S.
4,753,935). The mixture of Mycophenolic acid (32.0 g), thionyl chloride (25.0
ml) and DMF (0.3 ml) in dichloromethane (250 ml) was stirred at room
temperature for 3 hours, after which the volatile components were removed
under vacuum to afford mycophenolic acid chloride as an oil. The
mycophenolic acid chloride oil was dissolved in dichloromethane (50.0 ml) and
added to the chilled solution of morpholinoethanol (30.5 ml) in
dichloromethane (250 ml). After stirring for 90 min at 4°C, the reaction
mixture was washed with water and then with aqueous sodium bicarbonate.
The organic solution was dried with sodium sulfate and evaporated to yield
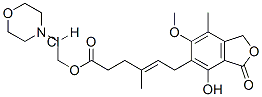
Mycophenolate mofetil: morpholinoethyl E-6-(1,3-dihydro-4-hydroxy-6-
methoxy-7-methyl-3-oxo-5-isobenzofuranyl)-4-methyl-4-hexenoate (melting
point 93-94°C).
The product (38.0 g) was dissolved in isopropanol (200 ml) and the solution
was added to a solution of hydrogen chloride (10.0 g) in isopropanol (150 ml).
The hydrochloride of Mycophenolate mofetil was collected by filtration and
dried under vacuum (melting point 154-155°C).