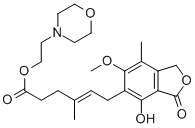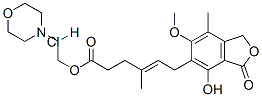
2-morpholin-4-ylethyl (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1H-isobenzofuran-5-yl)-4-methyl-hex-4-enoate hydrochloride
- Product Name2-morpholin-4-ylethyl (E)-6-(4-hydroxy-6-methoxy-7-methyl-3-oxo-1H-isobenzofuran-5-yl)-4-methyl-hex-4-enoate hydrochloride
- CAS116680-01-4
- CBNumberCB91564362
- MFC23H32ClNO7
- MW0
- MDL NumberMFCD28168033
- MOL File116680-01-4.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 154-155 °C |
| storage temp. | Store at -20°C |
| solubility | >83.3mg/mL in DMSO |
| form | Powder |
| NCI Dictionary of Cancer Terms | CellCept |
| FDA UNII | UXH81S8ZVB |
| NCI Drug Dictionary | Cellcept |