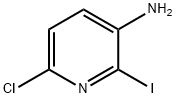
Step a: Synthesis of 6-chloro-2-iodopyridin-3-amine
To a solution of 6-chloropyridin-3-amine (10.0 g, 77.8 mmol) in ethanol (150 mL) was sequentially added silver sulfate (12.1 g, 38.9 mmol) and iodine (23.7 g, 93.4 mmol). The reaction mixture was stirred at 20 °C overnight. After completion of the reaction, the solvent was removed by distillation under reduced pressure. Water (100 mL) and ethyl acetate (200 mL) were added to the residue and the organic layer was separated. The aqueous layer was extracted three times with ethyl acetate (100 mL x 3). The organic layers were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to obtain the crude product. The crude product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate=7:1) to afford 6-chloro-2-iodopyridin-3-amine (17.1 g, 86% yield).
1H NMR (DMSO, 300MHz) δ 7.16 (d, J = 8.4Hz, 1H), 7.01 (d, J = 8.4Hz, 1H), 5.57 (s, 2H).