Sepracor’s Brovana®, a nebulized long acting bronchodilator,
was launched in the U.S. in April 2007. The β2-
adrenoceptor agonist is indicated for the twice-daily, longtermmaintenance
treatment of bronchoconstriction in patients with chronic obstructive pulmonary disease (COPD), which
includes chronic bronchitis and emphysema. It is the first
long-acting nebulized bronchodilator approved by the FDA
for this indication.
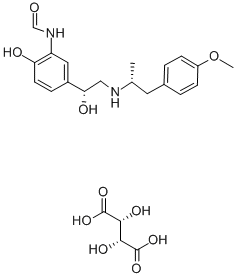
Aformoterol is the (R,R)-enantiomer of the β2-adrenergic receptor (β2-AR) agonist formoterol . It selectively binds to β1- over β2-ARs (Kds = 2.9 and 113 nM, respectively) as well as β3-adrenergic, B2 bradykinin, neurokinin 1 (NK1) and NK2 receptors when used at concentrations up to 3 μM. Aformoterol induces cAMP accumulation in cultured human bronchial epithelial cells. Ex vivo, aformoterol (0.01-1,000 nM) induces dose-dependent relaxation of guinea pig tracheal strips precontracted with carbamoylcholine , ovalbumin, or histamine (pD2s = 8.4, 9.5, and 9.5, respectively). In vivo, aformoterol reverses histamine- and ovalbumin-induced bronchoconstriction in guinea pigs (ED50s = 1 and 40 nmol/kg, respectively). Formulations containing aformoterol have been used in the treatment of chronic obstructive pulmonary disease (COPD).
Anti-asthmatic
and bronchodilator.
Arformoterol Tartrate, can be used in the synthesis of Omeprazole (O635000), which is a proton pump inhibitor, that inhibits gasteric secretion, also used in the treatment of dyspepsia, peptic ulcer disease, etc. It is also the impurity of Esomeprazole Magnesium (E668300), which is the S-form of Omeprazole, and is a gastric proton-pump inhibitor. Also, It can be used for the preparation of olodaterol, a novel inhaled β2-adrenoceptor agonist with a 24h bronchodilatory efficacy.
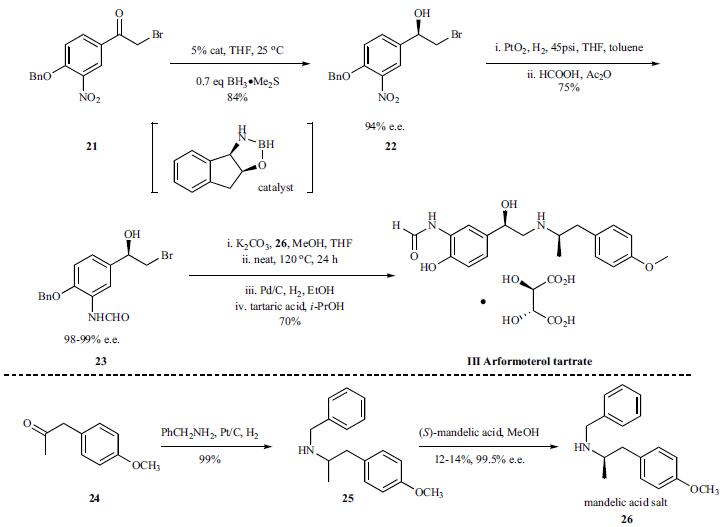
There are several reports on the synthesis
of arformoterol. A large-scale synthesis of
enantio/diastereomerically pure (R,R)-formoterol is cited
here. Bromoalcohol 22 was synthesized in
84% yield with 94% e.e. through the catalytic enantioselective
reduction of bromo ketone 21. The nitro functional
group in 22 was reduced in quantitative yield by hydrogena-tion in the presence of Adams catalyst and the resulting aniline
was isolated by filtration of the catalyst and removal of
the solvent. In order to avoid auto-oxidation, the aniline was
treated with a mixture of formic acid and acetic anhydride
immediately after the removal of the platinum catalyst. Upon
concentrating the reaction mixture, bromohydrin 23 crystallized
and could be isolated in 75% yield with 98.6% e.e. It
was further enriched to >99.5% e.e. by a single re-crystallization
from ethylacetate. Next, a mixture of bromohydrin
23 and amine salt (R)-26-(S)-mandelic acid was treated with
K2CO3 resulting in generation of the corresponding epoxide
of 23 and liberation of the free base of (R)-26. After an
aqueous work up to remove salts and mandelic acid, the reaction
mixture was heated to 120??C to affect epoxide opening
with the amine of 26. Removal of the benzyl protecting
groups of the resulting crude product via catalytic hydrogenation
followed by salt formation with tartaric acid afforded
arformoterol tartrate (III) in 70% yield upon crystallization.