Amikacin is made semisynthetically from kanamycin A. Interestingly, the L-hydroxyaminobutyryl amide
(HABA) moiety attached to N-3 inhibits adenylation and phosphorylation in the distant amino sugar ring (at
C-2′and C-3′), even though the HABA substituent is not where the enzymatic reaction takes place. This
effect is attributed to decreased binding to the R factor–mediated enzymes.
Amikacin is a semi-synthetic derivative of kanamycin. It is much less sensitive to the enzymes that inactivate aminoglycoside antibiotics. The spectrum is similar to that of gentamicin. Amikacin principally finds use in the treatment of infections arising from bacteria that are resistant to gentamicin and/or tobramycin.
Antibacterial;Ribosomal protein synthesis inhibitor
Amikacin is highly effective with respect to Gram-negative microorganisms (blue-pus
and gastric bacilli, rabbit fever, serratia, providencia, enterobacteria, proteus, salmonella,
shigella), as well as Gram-positive microorganisms (staphylococci, including those that
are resistant to penicillin and some cephalosporins), and a few strains of streptococci.
It is used for severe bacterial infections: peritonitis, sepsis, meningitis, osteomyelitis,
endocarditis, pneumonia, pleural empyema, pulmonary abscess, purulent skin and soft tissue infections, and infections of the urinary tract that are caused by microorganisms sensitive to the drug. Synonyms of this drug are amikin, biklin, novamin, and others.
ChEBI: An amino cyclitol glycoside that is kanamycin A acylated at the N-1 position by a 4-amino-2-hydroxybutyryl group.
Preparation of L-(-)-γ-benzyloxycarbonylamino-α-hydroxybutyric acid: L-(-)-γ-
amino-α-hydroxybutyric acid (7.4 g, 0.062 mol) was added to a solution of
5.2 grams (0.13 mol) of sodium hydroxide in 50 ml of water. To the stirred
solution was added dropwise at 0-5°C over a period of 0.5 hour, 11.7 grams
(0.068 mol) of carbobenzoxy chloride and the mixture was stirred for another
hour at the same temperature. The reaction mixture was washed with 50 ml
of ether, adjusted to pH 2 with dilute hydrochloric acid and extracted with four
80 ml portions of ether. The ethereal extracts were combined, washed with a
small amount of saturated sodium chloride solution, dried with anhydrous
sodium sulfate and filtered. The filtrate was evaporated in vacuum and the
resulting residue was crystallized from benzene to give 11.6 grams (74%) of
colorless plates; MP 78.5°C to 79.5°C.
Preparation of N-Hydroxysuccinimide Ester of L-(-)-γ-Benzyloxycarbonylamino-
α-hydroxybutyric acid: A solution of 10.6 grams (0.042 mol) of L-(-)-γ-
benzyloxycarbonylamino-α-hydroxybutyric acid and 4.8 grams (0.042 mol) of
N-hydroxysuccinimide in 200 ml of ethyl acetate was cooled to 0°C and then
8.6 grams (0.042 mol) of dicyclohexylcarbodiimide was added. The mixture
was kept overnight in a refrigerator. The dicyclohexylurea which separated
was filtered off and the filtrate was concentrated to about 50 ml under
reduced pressure to give colorless crystals of L-(-)-γ-benzyloxycarbonylamino-
α-hydroxybutyric acid which were collected by filtration; 6.4 grams, MP 121-
122.5°C. The filtrate was evaporated to dryness in vacuum and the crystalline
residue was washed with 20 ml of a benzene-n-hexane mixture to give an
additional amount of L-(-)-γ-benzyloxycarbonylamino-α-hydroxybutyric acid.
The total yield was 13.4 grams (92%).
Preparation of 1-[L-(-)-γ-Benzyloxycarbonylamino-α-Hydroxybutyryl]-6'-
Carbobenzoxykanamycin A: A solution of 1.6 grams (4.6 mmol) of L-(-)-γ-
benzyloxycarbonylamino-α-hydroxybutyric acid in 40 ml of ethylene glycol
dimethyl ether (DME) was added dropwise to a stirred solution of 2.6 grams
(4.2 mmol) of 6'-monobenzyloxycarbonylkanamycin A in 40 ml of 50%
aqueous ethylene glycol dimethyl ether and the mixture was stirred overnight.
The reaction mixture was evaporated under reduced pressure to give a brown
residue 1-[L-(-)-γ-benzyloxycarbonylarnino-α-hydroxybutyryl]-6'-
carbobenzoxykanamycin A which was used for the next reaction without
further purification.
Preparation of 1-[L-(-)-γ-Amino-α-Hydroxybutyryl] Kanamycin A: The crude
product 1-[L-(-)-γ-benzyloxycarbonylamino-α-hydroxybutyryl]-6'-
carbobenzoxykanamycin A was dissolved in 40 ml of 50% aqueous dioxane
and a small amount of insoluble material was removed by filtration. To the
filtrate was added 0.8 ml of glacial acetic acid and 1 gram of 10% palladiumon-
charcoal and the mixture was hydrogenated at room temperature for 24
hours in a Parr hydrogenation apparatus. The reaction mixture was filtered to
remove the palladium catalyst and the filtrate was evaporated to dryness in
vacuum.
The residue was dissolved in 30 ml of water and chromatographed on a column of CG-50 ion exchange resin (NH4
+ type, 50 cm x 1.8 cm). The
column was washed with 200 ml of water and then eluted with 800 ml of 0.1
N NH4OH, 500 ml of 0.2 N NH4OH and finally 500 ml of 0.5 N NH4OH. Ten
milliliter fractions were collected and fractions 146 to 154 contained 552 mg
(22%. based on carbobenzoxykanamycin A, 6'-
monobenzyloxycarbonylkanamycin A) of the product which was designated
BB-K8 lot 2. MP 187°C (dec). Relative potency against B. subtilis (agar plate)
= 560 mcg/mg (standard: kanamycin A free base).
A solution of 250 mg of BB-K8 lot 2 in 10 ml of water was subjected to
chromatography on a column of CG-50 (NH4
+ type, 30 cm x 0.9 cm). The
column was washed with 50 ml of water and then eluted with 0.2 N NH4OH.
Ten milliliter fractions were collected. Fractions 50 to 63 were combined and
evaporated to dryness under reduced pressure to give 98 mg of the pure
product base.
Preparation of the Monosulfate Salt of 1-[L-(-)-γ-Amino-α-Hydroxybutyryl]
Kanamycin A: One mol of 1-[L-(-)-γ-amino-α-hydroxybutyryl] kanamycin A is
dissolved in 1 to 3 liters of water. The solution is filtered to remove any
undissolved solids. To the chilled and stirred solution is added one mol of
sulfuric acid dissolved in 500 ml of water. The mixture is allowed to stir for 30
minutes, following which cold ethanol is added to the mixture till precipitation
occurs. The solids are collected by filtration and are determined to be the
desired monosulfate salt.
Among other organisms, Acinetobacter,
Alkaligenes, Campylobacter, Citrobacter, Hafnia, Legionella,
Pasteurella, Providencia, Serratia and Yersinia spp. are usually
susceptible in vitro. Stenotrophomonas maltophilia, many nonaeruginosa
pseudomonads and Flavobacterium spp. are resistant.
M. tuberculosis (including most streptomycin-resistant
strains) and some other mycobacteria (including M. fortuitum
and the M. avium complex) are susceptible; most other mycobacteria,
including M. kansasii, are resistant. Nocardia asteroides
is susceptible.
It exhibits typical aminoglycoside characteristics, including
an effect of divalent cations on its activity against Ps. aeruginosa
analogous to that seen with gentamicin and synergy with
β-lactam antibiotics.
Amikacin is unaffected by many of the modifying enzymes
that inactivate gentamicin and tobramycin and is consequently active against staphylococci,
enterobacteria and Pseudomonas that owe their resistance
to the production of those enzymes. However, AAC(6′),
ANT(4′) and some forms of APH(3′) can confer resistance;
because these enzymes generally do not confer gentamicin
resistance, amikacin-resistant strains can be missed in routine
susceptibility tests when gentamicin is used as the representative
aminoglycoside.
There have been reports of resistance arising during treatment
of infections due to Serratia spp. and Ps. aeruginosa.
Outbreaks of infection with multiresistant strains of enterobacteria
and Ps. aeruginosa have occurred after extensive use,
particularly in burns units. Bacteria that owe their resistance
to the expression of ANT(4′) have been described in Staph.
aureus, coagulase-negative staphylococci, Esch. coli, Klebsiella
spp. and Ps. aeruginosa. In E. faecalis, resistance to penicillin–
aminoglycoside synergy has been associated with plasmidmediated
APH(3′). Resistance in Gram-negative organisms is
usually caused by either reduced accumulation of the drug or,
more commonly, by the aminoglycoside-modifying enzymes
AAC(6′) or AAC(3)-VI. The latter enzyme is usually found in
Acinetobacter spp., but has also been found, encoded by a transposon,
in Prov. stuartii. One type of AAC(6) is chromosomally
encoded by Ser. marcescens, though not usually expressed.
The prevalence of resistance to amikacin remains low
(<5%) in many countries but can change rapidly with
increased usage of the drug. However, the spread of extended
spectrum β-lactamases belonging to the TEM and SHV families
may result in an increase in amikacin resistance that is
not associated with use, since most strains that produce such
enzymes also produce AAC(6′).
Amikacin was synthesized by Kawaguchi et al. of the Bristol-Banyu Research Institute in 1970 starting with kanamycin and the acyl moiety of butirosin. Its design is based on knowledge of the mechanisms of bacterial resistance to kanamycin and related compounds in which the 3 -hydroxyl group of the antibiotic is phosphorylated enzymatically. The acyl moiety in butirosin prevents this enzymatic inactivation.
Cmax 7.5 mg/kg intramuscular: c. 30 mg/L after 1 h
500 mg 30-min infusion: 35–50 mg/L end infusion
15 mg/kg 30-min infusion: >50 mg/L after 1 h
Plasma half-life: 2.2 h
Volume of distribution: 0.25–0.3 L/kg
Plasma protein binding: 3–11%
It is readily absorbed after intramuscular administration.
Rapid intravenous injection of 7.5 mg/kg produced concentrations
in excess of 60 mg/L shortly after injection.
Most pharmacokinetic parameters follow an almost linear
correlation when the once-daily doses (15 mg/kg) are compared
with the traditional 7.5 mg/kg twice daily. In patients on CAPD,
there was no difference in mean peak plasma concentration or
volume of distribution whether the drug was given intravenously or intraperitoneally. However, in patients with significant burn
injuries, doses should be increased to 20 mg/kg.
In infants receiving 7.5 mg/kg by intravenous injection,
peak plasma concentrations were 17–20 mg/L. No accumulation
occurred on 12 mg/kg per day for 5–7 days. There was
little change in the plasma concentration or the half-life (1.7
and 1.9 h) on the third and seventh days of a period over
which 150 mg/m2 was infused over 30 min every 6 h. When
the dose was raised to 200 mg/m2 the concentration never fell
below 8 mg/L. The plasma half-life was longer in babies of
lower birth weight and was still 5–5.5 h in babies aged 1 week
or older. The importance of dosage control in the neonate is
emphasized by the findings that there is an inverse relationship
between post-conception age and plasma elimination
half-life, though in extremely premature babies the weight of
the child is also a significant predictor of half-life.
Severe infection (including septicemia, neonatal sepsis, osteomyelitis,
septic arthritis, respiratory tract, urinary tract, intra-abdominal, peritoneal
and soft tissue infections) caused by susceptible micro-organisms
Sepsis of unknown origin (combined with a β-lactam or anti-anaerobe
agent as appropriate).
Mycobacterial infection
Amikacin is principally used for the treatment of infections
caused by organisms resistant to other aminoglycosides
because of their ability to degrade them. Peak concentrations
on 15 mg/kg once daily administration should exceed
45 mg/L, and trough concentration of <5 mg/L should be
maintained to achieve therapeutic effects.
Amikacin, 1-N-amino-α-hydroxybutyrylkanamycin A(Amikin), is a semisynthetic aminoglycoside first preparedin Japan. The synthesis formally involves simple acylationof the 1-amino group of the deoxystreptamine ring ofkanamycin A with L-AHBA. This particular acyl derivativeretains about 50% of the original activity of kanamycin Aagainst sensitive strains of Gram-negative bacilli. The LAHBAderivative is much more active than the D-isomer.The remarkable feature of amikacin is that it resists attackby most bacteria-inactivating enzymes and, therefore, is effectiveagainst strains of bacteria that are resistant to otheraminoglycosides, including gentamicin and tobramycin.In fact, it is resistant to all known aminoglycoside-inactivatingenzymes, except the aminotransferase that acetylates the6 amino group and the 4'-nucleotidyl transferase thatadenylylates the 4'-hydroxyl group of aminoglycosides.
Preliminary studies indicate that amikacin may be lessototoxic than either kanamycin or gentamicin. Higherdosages of amikacin are generally required, however, for the treatment of most Gram-negative bacillary infections. Forthis reason, and to discourage the proliferation of bacterialstrains resistant to it, amikacin currently is recommended forthe treatment of serious infections caused by bacterialstrains resistant to other aminoglycosides.
Distribution
The apparent volume of distribution indicates distribution
throughout the extracellular water. Following an intravenous
bolus of 0.5 g, peak concentrations in blister fluid were around
12 mg/L, with a mean elimination half-life of 2.3 h. In patients
with impaired renal function, penetration and peak concentration
increased linearly with decrease in creatinine clearance.
In patients with purulent sputum, a loading dose of 4 mg/kg
intravenously plus 8 h infusions of 7–12 mg/kg produced sputum
concentrations around 2 mg/L, with a mean sputum:serum
ratio of 0.15. With brief infusions over 10 min for 7 days, sputum
concentrations of around 9% of the simultaneous serum
values have been found.
Concentrations in the CSF of adult volunteers receiving
7.5 mg/kg intramuscularly were less than 0.5 mg/L and virtually
the same in patients with meningitis. Rather higher, but
variable, concentrations up to 3.8 mg/L have been found in
neonatal meningitis.
Amikacin crosses the placenta, and concentrations of
0.5–6 mg/L have been found in the cord blood of women
receiving 7.5 mg/kg in labor. Concentrations of 8 mg/L and
16.8 mg/L were reached in the fetal lung and kidney, respectively,
after a standard dose of 7.5 mg/kg given to healthy
women before therapeutic abortion.
Excretion
Only 1–2% of the administered dose is excreted in the bile,
with the remainder excreted in the urine, producing urinary
concentrations of 150–3000 mg/L. Renal clearance is
70–84 mL/min, and this, with the ratio of amikacin to creatinine
clearance (around 0.7), indicates that it is filtered and
tubular reabsorption is insignificant. Accumulation occurs in
proportion to reduction in renal function, although there may
be some extrarenal elimination in anephric patients. The mean
plasma half-life in patients on hemodialysis was around 4 h,
while that on peritoneal dialysis was 28 h.
In patients receiving 500 mg/kg preoperatively, concentrations
in gallbladder wall reached 34 mg/L and in bile 7.5 mg/L in some patients. In patients given 500 mg intravenously 12 h
before surgery and 12 hourly for four doses thereafter, the
mean bile:serum ratio 1 h after the dose was around 0.4.
Ototoxicity
Neurosensory hearing loss (mainly high-tone deafness) and
labyrinthine injury have been detected, but have seldom been
severe. High-frequency hearing loss and vestibular impairment
have been described in about 5% of patients and conversational
loss in about 0.5%; more in patients monitored
audiometrically (29%) and by caloric testing (19%).
Patients with high-tone hearing loss have generally received
more drug and for longer than patients without; in patients
receiving long-term treatment for tuberculosis no other factors
were associated with the development of ototoxicity. On multiple
daily dosing, over half the patients with peak serum concentrations
exceeding 30 mg/L or trough concentrations exceeding
10 mg/L developed cochlear damage; here, the main contributory
factor was previous treatment with other aminoglycosides.
Nephrotoxicity
Impairment of renal function, usually mild or transient, has
been observed in 3–13% of patients, notably in the elderly or
those with pre-existing renal disorders or treated concurrently
or previously with other potentially nephrotoxic agents.
Other reactions
Adverse effects common to aminoglycosides occur, including
hypersensitivity, gastrointestinal disturbances, headache,
drug fever, peripheral nervous manifestations, eosinophilia,
mild hematological abnormalities and disturbed liver function
tests without other evidence of hepatic derangement.
Poison by intravenous,intraperitoneal, and intramuscular routes. Moderately toxicby intraperitoneal route. An experimental teratogen. Whenheated to decomposition it emits toxic fumes of NOx.
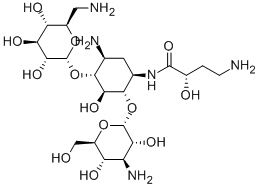
Amikacin, O-3-amino-3-deoxy-α-D-glucopyranosyl-(1→4)-O-[6-amino-6-
deoxy-α-D-glucopyranosyl-(1→6)]-N3
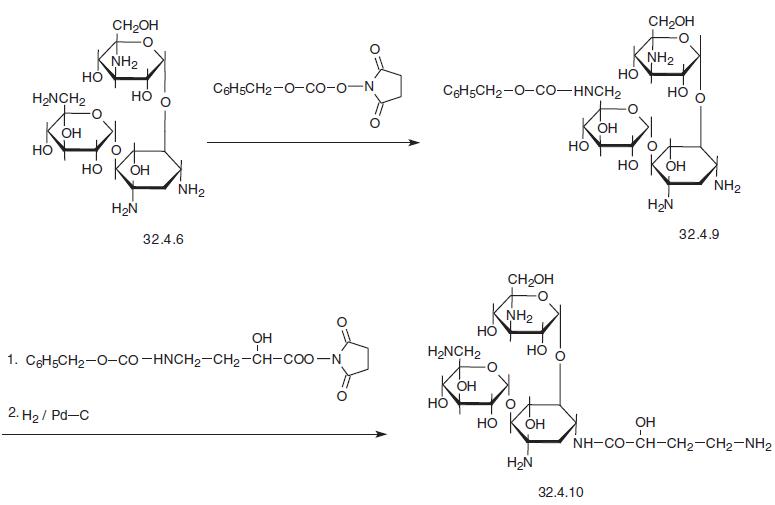
-(4-amino-L-2-hydroxybutyryl)-2-deoxy-L-strepta�mine (3.4.10), is a semisynthetic antibiotic that is synthesized from kanamycin (3.4.6). The
primary amino group in this molecule is previously protected by acylating it with N-
(benzoyloxycarbonyloxy) succinimide in dimethylformamide, after which the resulting
product (32.4.9) is treated with an ester synthesized from N-hydroxysuccinimide and ben�zyloxycarbonylamino-α-l-() hydroxybutyric acid, and as a result the 4-amino group of
the streptamine region of the molecule is selectively acylated. Further removal of two ben�zyloxycarbonylamine protective groups in the traditional manner, via hydrogen reduction
using a palladium on carbon catalyst, forms the desired amikacin (32.4.10).
Potentially hazardous interactions with other drugs
Antibacterials: increased risk of nephrotoxicity
with colistimethate or polymyxins and possibly
cephalosporins; increased risk of ototoxicity and
nephrotoxicity with capreomycin or vancomycin.
Ciclosporin: increased risk of nephrotoxicity.
Cytotoxics: increased risk with platinum compounds
of nephrotoxicity and possibly of ototoxicity
Diuretics: increased risk of ototoxicity with loop
diuretics.
Muscle relaxants: enhanced effects of non�depolarising muscle relaxants and suxamethonium.
Parasympathomimetics: antagonism of effect of
neostigmine and pyridostigmine.
Tacrolimus: increased risk of nephrotoxicity.
Amikacin diffuses readily through extracellular fluids
and has been found in cerebrospinal fluid, pleural fluid,
amniotic fluid and in the peritoneal cavity following
parenteral administration. It is excreted in the urine
unchanged, primarily by glomerular filtration.