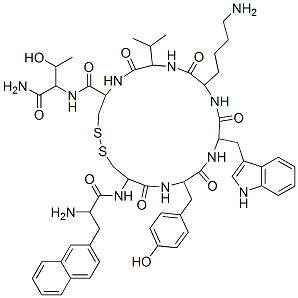
Lanreotide acetate, an octapeptide somatostatin analog, reached its first
worldwide market in France for acromegaly when surgery or radiotherapy have
failed to restore normal growth hormone secretion. Lanreotide is a selective inhibitor of
growth hormone and reduces the secretion of growth hormone, thyrotropin, motilin and
pancreatic polypeptide in humans. Lanreotide has antiproliferative properties and is
reportedly in clinical trials for the prevention of restenosis following coronary artery
angioplasty, for diabetic retinopathy, and as a therapy for psoriasis. Its potential for
neuroendocrine tumors and hormone-responsive prostate cancer has also been
demonstrated.
Lanreotide acetate has been used as an inhibitor to test the plasticity of hydrogen sulfide modulation by growth hormone/thyroid hormone signaling in wild-type mice. It is a drug used in the control and prevention of glycemia and hypoglycemic brain damage without surgery in patients diagnosed with congenital hyperinsulinism. It improves outcomes in patients suffering from acromegalyresulting from invasive pituitary macroadenoma.
Lanreotide acetate is a somatostatin analog, a selective agonist for the SRIF-1 sst2 subtype of somatostatin receptor with a binding affinity of 0.8 nM for sst2 compared to 5.2 nM for sst5, 100 nM for sst3 and more than 1000 nM for the SRIF-2 subtypes, sst1 and sst4 receptors. It is used clinically in the management of acromegaly and symptoms caused by neuroendocrine tumors, and in recent studies can also inhibit tumor growth and has shown activity against non-endocrine tumors.
Lanreotide is used in the treatment of acromegaly, due to both pituitary and non-pituitary growth hormone-secreting tumors, and the management of symptoms caused by neuroendocrine tumors, particularly carcinoid tumors and VIPomas. Lanreotide (as lanreotide acetate) is manufactured by Ipsen, and marketed under the trade name Somatuline. It is available in several countries, including the United Kingdom, Australia and Canada, and was approved for sale in the United States by the Food and Drug Administration (FDA) on August 30, 2007. Lanreotide acetate is available in two formulations: a sustained release formulation (sold under the trade name Somatuline LA) and an extended release formulation (UK trade name Somatuline Autogel, or Somatuline Depot in the U.S.). In the United States and Canada, lanreotide is only indicated for the treatment of acromegaly. In the United Kingdom, it is also indicated in the treatment of thyrotrophic adenoma, a rare tumor of the pituitary gland which secretes TSH. Interestingly, lanreotide also shows activity against non-endocrine tumors, and, along with other somatostatin analogues, is being studied as a possible general antitumor agent.
The side effects of Lanreotide Acetate include: Blurred vision; chest pain or discomfort; dizziness; gaseous abdominal or stomach pain; headache; lightheadedness, dizziness, or fainting; nervousness; pale skin; pounding in the ears; recurrent fever; slow, fast, or irregular heartbeat; stomach fullness; troubled breathing with exertion; unusual bleeding or bruising; unusual tiredness or weakness; yellow eyes or skin.