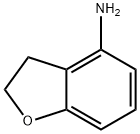
Synthesis of 4-amino-2,3-dihydrobenzofuran (1c): compound 1b (4.10 g, 16.3 mmol) was dissolved in 48% aqueous hydrobromic acid solution (20.0 mL) and mixed with thorough stirring. The reaction mixture was heated to reflux at 110°C for 16 hours. Upon completion of the reaction, the mixture was cooled to room temperature, followed by the slow addition of sodium hydroxide pellets in an ice bath at 0°C to adjust the pH to about 9. The reaction mixture was extracted several times with ethyl acetate (50 mL × 4). The organic phases were combined, dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure to give the crude product. Purification of the crude product by medium pressure column chromatography (Smart Flash EPCLC W-Prep 2XY system, eluent: hexane/ethyl acetate = 1/1) afforded 4-amino-2,3-dihydrobenzofuran (Compound 1c) (1.49 g, 11.0 mmol, 67.7% yield) as a colorless solid. tlc Rf = 0.30 (unfolding agent: Hexane/ethyl acetate = 1/1).1H NMR (400 MHz, CDCl3) δ 6.94 (dd, J = 8.4, 8.4 Hz, 1H), 6.28 (dd, J = 0.4, 7.6 Hz, 1H), 6.23 (dd, J = 0.4, 7.6 Hz, 1H), 4.59 (t, J = 8.4 Hz, 2H), 3.60 ( br s, 2H), 3.02 (t, J = 8.4 Hz, 2H).
![N-[2-(2-hydroxyethyl)-3-Methoxyphenyl]-2,2-
diMethylpropanaMide](/CAS/GIF/76093-72-6.gif)