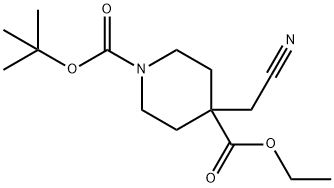
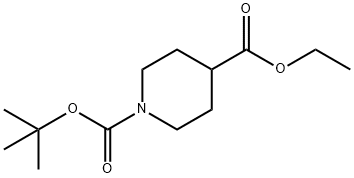
A) Synthesis of 4-tert-butyl 4-ethyl ester of 4-(cyanomethyl)piperidine-1,4-dicarboxylate: To a mixed solution of diisopropylamine (4.7 g) and THF (75 mL) was slowly added hexane solution of n-butyllithium (1.6 M, 29 mL) under cooling in an ice bath and stirred for 30 min. Subsequently, a mixed solution of 4-tert-butyl 4-ethylpiperidine-1,4-dicarboxylate (6.0 g) and THF (10 mL) was added dropwise to the reaction system at the same temperature and stirring was continued for 3 hours. Next, 2-bromoacetonitrile (5.6 g) was added and the reaction mixture was stirred under an ice bath for 12 hours. Upon completion of the reaction, the solvent was evaporated under reduced pressure. Water was added to the residue and extracted with ethyl acetate. The organic layer was dried with anhydrous magnesium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: ethyl acetate/hexane) to afford 4-(cyanomethyl)-1,4-piperidinedicarboxylic acid-1-(1,1-dimethylethyl)4-ethyl ester (2.6 g) as a white solid. The product was characterized by 1H NMR (300 MHz, DMSO-d6): δ 1.21 (3H, t, J=7.0 Hz), 1.39 (9H, s), 1.43-1.54 (2H, m), 1.90-2.00 (2H, m), 2.87 (2H, s), 2.96-3.12 (2H, m), 3.57-3.67 (2H, m), 4.11-4.22 (2H, m).