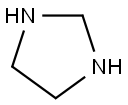
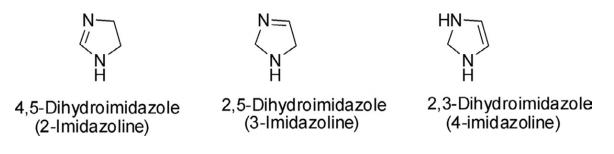
Imidazolidine is produced by a cyclocondensation reaction between ethylenediamine and an aldehyde. The yield is 70 %. The reaction conditions are that one of the amino groups of ethylenediamine is present using the secondary amine form.
Synthesis of Imidazolidine derivatives including:
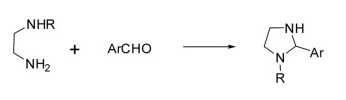
(1) Synthesis of 1,3-dibenzyl-2-arylimidazolidine
It is divided into three steps: the first step is the condensation of ethylenediamine with aldehyde in dry benzene to obtain N,N′-dibenzylidene-1,2-diamine, and the second step is the reduction of N,N′-dibenzylidene ethylenediamine to N,N′-dibenzylidene ethylenediamine in ethanol with sodium borohydride. The substituted diamine was condensed with an aryl aldehyde in the final step to give 1,3-dibenzyl-2-arylimidazolidine.
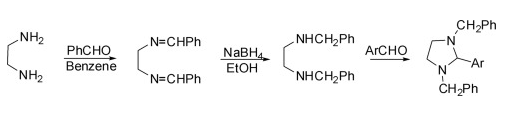
(2)Synthesis of 2-iminoimidazolidine
Method: Ethylenediamine reacts with cyanobromide to form 2-iminoimidazolidine by substitution-cyclisation.

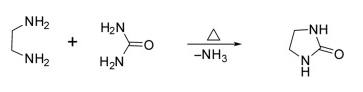
(3) Synthesis of Imidazolidin-2-one
Methods: Imidazolidin-2-one was prepared by heating ethylenediamine and urea with 75% yield.