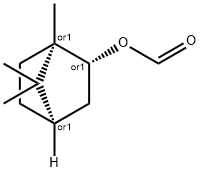
ISOBORNYL FORMATE has green-earthy, herbaceous-camphoraceous
odor, more piney than that of the Bornyl
formate, and more camphor-like, less nutlike.
Suggested for use in fruit flavors, and although this ester does have a sweet taste
similar to that of Bornyl formate, it is overall
less fruity or attractive.
The use in fruit flavors amounts to a few
ppm in the finished product (traces).
Isobornyl formate has a characteristic aromatic, pine needles odor.
May be prepared by reaction of formic acid with camphene in the
presence of a catalyst; also in the absence of a catalyst; the resulting products do not exhibit well-defined optical characteristics.
The corresponding optically active esters have been prepared from
d- or ι-camphene and formic acid in the presence of phthalic
anhydride.
Isobornyl formate has a characteristic aromatic, pine needles odor.
By reaction of formic acid with camphene in the presence of a catalyst; also in the absence of a catalyst; the resulting
products do not exhibit well-defined optical characteristics. The corresponding optically active esters have been prepared from d- or
l-camphene and formic acid in the presence of phthalic anhydride
ChEBI: Isobornyl formate is a bornane monoterpenoid that is isoborneol in which the hydroxy hydrogen has been replaced by a formyl group. It has a role as a plant metabolite. It is a bornane monoterpenoid, a bridged compound and a formate ester. It is functionally related to a borneol.