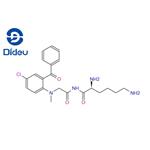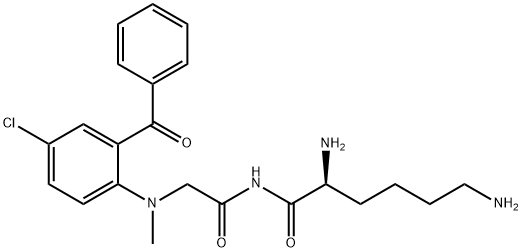
pro-diazepam
- Product Namepro-diazepam
- CAS65617-86-9
- MFC22H27ClN4O3
- MW430.933
- EINECS
- MOL File65617-86-9.mol
Chemical Properties
| Boiling point | 697.6±55.0 °C(Predicted) |
| Density | 1.253±0.06 g/cm3(Predicted) |
| pka | 13.65±0.46(Predicted) |
Usage And Synthesis
(a) 20.9 g of N-benzyloxycarbonylglycine were suspended in 1500 ml of dry
1,2-dimethoxyethane and the suspension was cooled to -20°C. 10.1 g of Nmethylmorpholine
and 13.7 g of isobutylchloroformate were added, the
resulting solution was stirred at -20°C for 1 hour and then filtered. The filtrate
was added portion-wise over a period of several hours to a refluxing solution
of 24.55 g of 5-chloro-2-methylaminobenzophenone in 200 ml of 1,2-
dimethoxyethane, the resulting mixture was boiled overnight and then
evaporated to dryness in vacuum. The yellow residue was dissolved in ethyl
acetate, washed with two portions of water and one portion of saturated
sodium chloride solution, dried over anhydrous magnesium sulfate and then
evaporated. Column chromatography of the residue on Florisil (Trade Mark)
using mixtures of benzene and chloroform yielded 35 g (80%) of pure 2-(Nbenzyloxycarbonylamino)-
N-(2-benzoyl-4-chlorophenyl)-N-methylacetamide as
a pale yellow gum.
43.7 g of 2-(N-benzyloxycarbonylamino)-N-(2-benzoyl-4-chlorophenyl)-Nmethylacetamide were dissolved in 200 ml of a 30% solution of hydrogen bromide in glacial acetic acid and the resulting solution was stirred overnight at room temperature. The mixture was added slowly to a large excess (2000 ml) of dry diethyl ether with vigorous stirring. The product, which separated was allowed to settle and the supernatant liquors were decanted off. The residue was triturated with 150 ml of acetone and the product filtered off, washed consecutively with the minimum amount of acetone and dry diethyl ether and dried in vacuum to give 29.5 g (77%) of 2-amino-N-(2-benzoyl-4- chlorophenyl)-N'-methylacetamide hydrobromide as a white hygroscopic powder of melting point 194°-195°C (decomposition).
(b) 84 g of N-benzyloxycarbonylglycine were suspended in 500 ml of alcoholfree chloroform and the suspension was cooled to -200°C. The stirred suspension was treated portion-wise over a period of 15 minutes with 90 g of phosphorus pentachloride and the stirring was continued until a clear solution was obtained. At this point, the cold mixture was added dropwise over a period of 30 minutes to a cold (-5°C) vigorously stirred emulsion consisting of 82 g of 5-chloro-2-methylaminobenzophenone, 347 g of potassium bicarbonate, 700 ml of chloroform and 1400 ml of water. The resulting mixture was stirred for a further 1 hour at -5°C and then overnight at room temperature. The stirring was then discontinued and the liquid phases allowed to separate. The chloroform layer was washed three times with 500 ml of water each time and evaporated in VW to give 150.7 g of a viscous yellow gum which was shown by physical methods to be almost pure (above 95%) 2- (N-benzyloxycarbonylamino)-N-(2-benzoyl-4-chlorophenyl)-Nmethylacetamide. The product above obtained was dissolved in 650 ml of a 30% solution of hydrogen bromide in glacial acetic acid and treated in an identical manner to that described in part (a) to give 2-amino-N-(2-benzoyl- 4chlorophenyl)-N-methylacetamide hydrobromide in a 77% overall yield from 5-chloro-2-methylaminobenzophenone.
(c) 1 mole of Nα,Nε-bisbenzyloxycarbonyl-L-lysyl N-hydroxysuccinimide ester was dissolved in 50 ml of dry dimethylformamide. The resulting solution was cooled to -20°C and there were added 1 mole of 2-amino-N-(2-benzoyl-4- chlorophenyl)-N-methylacetamide hydrobromide followed by the dropwise addition of 1 mole of N-ethylmorpholine. The resulting mixture was vigorously stirred for 1 hour at -20°C and then overnight at room temperature. The solvent was evaporated in vacuum and the residue dissolved in a mixture of dichloromethane and water. The organic and aqueous layers were separated and the aqueous phase extracted with further portions of dichloromethane. The combined organic phases (250 ml) were washed three times with 50 ml of water each time, dried over anhydrous magnesium sulfate and evaporated in vacuum to give a yellow oily residue, which was shown by physical methods to consist of Nα,Nε-bisbenzyloxycarbonyl-L-lysyl-N-(2-benzoyl-4-chlorophenyl)- N-methylglycinamide as an almost colorless light-sensitive gum, [α]D 20= -9.3° (c=1 in ethanol).
Nα,Nε-Bisbenzyloxycarbonyl-L-lysyl-N-(2-benzoyl-4-chlorophenyl)-Nmethylglycinamide was converted using a 30% solution of hydrogen bromide in glacial acetic acid into L-lysyl-N-(2-benzoyl-4-chlorophenyl)-Nmethylglycinamide dihydrobromide which was obtained as a hygroscopic powder of melting point 145°C (decomposition), [α]D 20= + 15.6° (c=1 in water).
Treatment of the foregoing dihydrobromide in aqueous solution by passage over an excess of an anion-exchange resin such as 'Amberlite' IRA-401 in the chloride form followed by lyophilisation of the eluate gave, in quantitative yield, L-lysyl-N-(2-benzoyl-4-chlorophenyl)-N-methylglycinamide dihydrochloride (avizafone) as a hygroscopic white light-sensitive powder of melting point 125°-145°C (slow decomposition), [α]D 20= +19.3° (c= 1 in water).
43.7 g of 2-(N-benzyloxycarbonylamino)-N-(2-benzoyl-4-chlorophenyl)-Nmethylacetamide were dissolved in 200 ml of a 30% solution of hydrogen bromide in glacial acetic acid and the resulting solution was stirred overnight at room temperature. The mixture was added slowly to a large excess (2000 ml) of dry diethyl ether with vigorous stirring. The product, which separated was allowed to settle and the supernatant liquors were decanted off. The residue was triturated with 150 ml of acetone and the product filtered off, washed consecutively with the minimum amount of acetone and dry diethyl ether and dried in vacuum to give 29.5 g (77%) of 2-amino-N-(2-benzoyl-4- chlorophenyl)-N'-methylacetamide hydrobromide as a white hygroscopic powder of melting point 194°-195°C (decomposition).
(b) 84 g of N-benzyloxycarbonylglycine were suspended in 500 ml of alcoholfree chloroform and the suspension was cooled to -200°C. The stirred suspension was treated portion-wise over a period of 15 minutes with 90 g of phosphorus pentachloride and the stirring was continued until a clear solution was obtained. At this point, the cold mixture was added dropwise over a period of 30 minutes to a cold (-5°C) vigorously stirred emulsion consisting of 82 g of 5-chloro-2-methylaminobenzophenone, 347 g of potassium bicarbonate, 700 ml of chloroform and 1400 ml of water. The resulting mixture was stirred for a further 1 hour at -5°C and then overnight at room temperature. The stirring was then discontinued and the liquid phases allowed to separate. The chloroform layer was washed three times with 500 ml of water each time and evaporated in VW to give 150.7 g of a viscous yellow gum which was shown by physical methods to be almost pure (above 95%) 2- (N-benzyloxycarbonylamino)-N-(2-benzoyl-4-chlorophenyl)-Nmethylacetamide. The product above obtained was dissolved in 650 ml of a 30% solution of hydrogen bromide in glacial acetic acid and treated in an identical manner to that described in part (a) to give 2-amino-N-(2-benzoyl- 4chlorophenyl)-N-methylacetamide hydrobromide in a 77% overall yield from 5-chloro-2-methylaminobenzophenone.
(c) 1 mole of Nα,Nε-bisbenzyloxycarbonyl-L-lysyl N-hydroxysuccinimide ester was dissolved in 50 ml of dry dimethylformamide. The resulting solution was cooled to -20°C and there were added 1 mole of 2-amino-N-(2-benzoyl-4- chlorophenyl)-N-methylacetamide hydrobromide followed by the dropwise addition of 1 mole of N-ethylmorpholine. The resulting mixture was vigorously stirred for 1 hour at -20°C and then overnight at room temperature. The solvent was evaporated in vacuum and the residue dissolved in a mixture of dichloromethane and water. The organic and aqueous layers were separated and the aqueous phase extracted with further portions of dichloromethane. The combined organic phases (250 ml) were washed three times with 50 ml of water each time, dried over anhydrous magnesium sulfate and evaporated in vacuum to give a yellow oily residue, which was shown by physical methods to consist of Nα,Nε-bisbenzyloxycarbonyl-L-lysyl-N-(2-benzoyl-4-chlorophenyl)- N-methylglycinamide as an almost colorless light-sensitive gum, [α]D 20= -9.3° (c=1 in ethanol).
Nα,Nε-Bisbenzyloxycarbonyl-L-lysyl-N-(2-benzoyl-4-chlorophenyl)-Nmethylglycinamide was converted using a 30% solution of hydrogen bromide in glacial acetic acid into L-lysyl-N-(2-benzoyl-4-chlorophenyl)-Nmethylglycinamide dihydrobromide which was obtained as a hygroscopic powder of melting point 145°C (decomposition), [α]D 20= + 15.6° (c=1 in water).
Treatment of the foregoing dihydrobromide in aqueous solution by passage over an excess of an anion-exchange resin such as 'Amberlite' IRA-401 in the chloride form followed by lyophilisation of the eluate gave, in quantitative yield, L-lysyl-N-(2-benzoyl-4-chlorophenyl)-N-methylglycinamide dihydrochloride (avizafone) as a hygroscopic white light-sensitive powder of melting point 125°-145°C (slow decomposition), [α]D 20= +19.3° (c= 1 in water).
Preparation Products And Raw materials
pro-diazepam manufacturers
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine