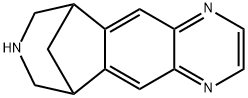
Varenicline, a partial agonist of the a4b2 nicotinic receptor, is a first-in-class
drug launched by Pfizer as an aid to smoking cessation treatment.
Varenicline exhibits dual action by decreasing craving and withdrawal symptoms,
and by decreasing the reinforcement associated with smoking. The addictive
properties of nicotine are thought to be mediated in part through its action as an agonist at α4β2 neuronal nicotinic acetylcholine receptors (nAChRs). Activation
of α4β2 receptors by nicotine increases the release of dopamine in the
mesolimbic system, an effect that is shared by most drugs of abuse. As nicotine
levels decrease, dopamine levels decline, which in turn stimulates the urge to
smoke. Additionally, a reduced dopaminergic tone due to abstinence from
smoking stimulates craving and the withdrawal syndrome. A partial agonist of
α4β2 receptors such as varenicline is expected to elicit a moderate and sustained
increase in dopamine levels to relieve craving and withdrawal symptoms. In
addition, by competitively binding to a4b2 receptors and inhibiting nicotineinduced
dopaminergic activation, a partial agonist could attenuate the pharmacologic
reward associated with smoking.
Smoking cessation (selective nicotinic receptor modulator).
Varenicline is a useful medication for smoking cessation.
ChEBI: An organic heterotetracyclic compound that acts as a partial agonist for nicotinic cholinergic receptors and is used (in the form of its tartate salt) as an aid to giving up smoking.
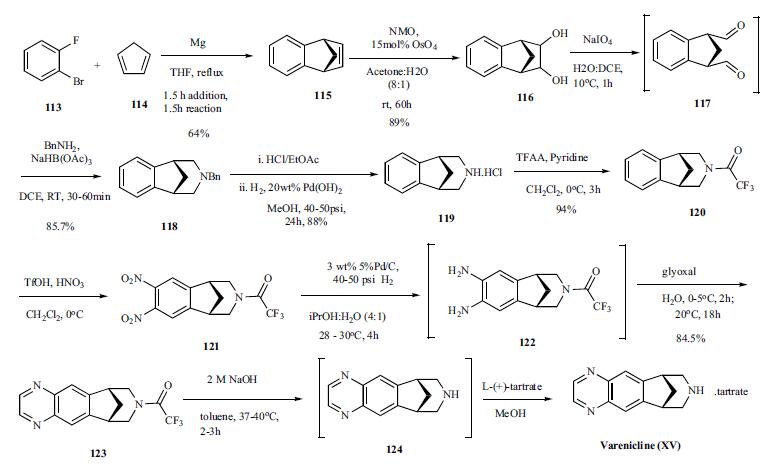
Several modifications to the original
synthesis have been reported in the literature, including
an improved process scale synthesis of the last few steps.The Grignard reaction was initiated on a
small scale by addition of 2-bromo fluorobenzene 113 to a
slurry of Magnesium turnings and catalytic 1,2-dibromoethane
in THF and heating the mixture until refluxing in
maintained. To this refluxing mixture was added a mixture
of the 2-bromo fluorobenzene 113 and cyclopentadiene 114
over a period of 1.5 h. After complete addition, the reaction
was allowed to reflux for additional 1.5 h to give the Diels-
Alder product 115 in 64% yield. Dihydroxylation of the olefin
115 by reacting with catalytic osmium tetraoxide in the
presence of N-methylmorpholine N-oxide (NMO) in acetone:
water mixture at room temperature provided the diol
116 in 89% yield. Oxidative cleavage of diol 116 with sodium
periodate in biphasic mixture of water: DCE at 10oC
provided di-aldehyde 117 which was immediately reacted
with benzyl amine in the presence of sodium acetoxyborohydride
to give benzyl amine 118 in 85.7% yield. The removal
of the benzyl group was effected by hydrogenation of
the HCl salt in 40-50 psi hydrogen pressure with 20%
Pd(OH)2 in methanol to give amine hydrochloride 119 in
88% yield. Treatment of amine 119 with trifluoroacetic anhydride
and pyridine in dichloromethane at 0oC gave trifluoroacetamide
120 in 94% yield. Dinitro compound 121 was
prepared by addition of trifluoroacetamide 120 to a mixture
of trifluoromethane sulfonic acid and nitric acid, which was
premixed, in dichloromethane at 0oC. Reduction of the dinitro
compound 121 by hydrogenation at 40-50 psi hydrogen
in the presence of catalytic 5%Pd/C in isopropanol:water
mixture provided the diamine intermediate 122 which was
quickly reacted with glyoxal in water at room temperature
for 18h to give compound 123 in 85% overall yield. The
trifluoroacetamide 123 was then hydrolyzed with 2 M sodium
hydroxide in toluene at 37-40oC for 2-3h followed by
preparation of tartrate salt in methanol to furnish varenicline
tartrate (XV).
Varenicline undergoes minimal metabolism with less
than 10
% excreted as metabolites. About 92
% of a dose is
excreted unchanged in the urine.
Minor metabolites in urine include varenicline
N-carbamoylglucuronide, N-glucosylvarenicline and
hydroxyvarenicline. In circulation, varenicline comprises
91
% of drug-related material.
[1] garrison gd, dugan se. varenicline: a first-line treatment option for smoking cessation. clin ther. 2009 mar;31(3):463-91.