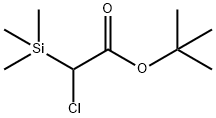
Tert-butyl-2-chloro-2-trimethylsilylacetate can olefinate enolizable ketones via the Peterson enolation reaction to generate functionalized tetrasubstituted olefins (α,β-unsaturated esters).
Tert-butyl-2-chloro-2-trimethylsilylacetate is prepared from t-Butyl Chloroacetate by sequential reaction with Lithium Diisopropylamide and Chlorotrimethylsilane followed by aqueous workup and distillation.
As this material is an activated alkyl halide, it must be considered a potential alkylating agent and lachrymator and handled with care and suitable protective clothing (e.g. gloves) in a fume hood.