Colorless to yellowish crystalline solid. Starts
turning brown at approximately 165℃; turns blackish-
brown at 187℃.
Riddelliine-containing plants are not used for food in the United States, and riddelliine and riddelliine N-oxide have no known commercial uses. However, the riddelliine-containing plant Senecio longilobus has been used in medicinal herb preparations in the United States, and S. jacobaea and S. vulgaris, both of which have been shown to contain riddelliine, are used in medicinal preparations in other parts of the world (Mattocks 1986).
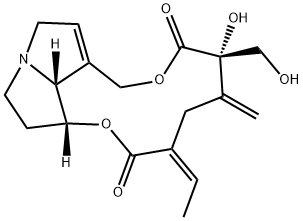
ChEBI: A macrolide that is 13,19-didehydrosenecionan bearing two additional hydroxy substituents at positions 12 and 18 as well as two additional oxo groups at positions 11 and 16.
Colorless to off-white crystalline solid. Starts turning brown at approximately 329°F; is blackish-brown at melting point.
Exposure to light and air can cause gradual oxidation. Insoluble in water.
riddelline reacts readily with oxidizing agents (slowly with atmospheric oxygen). On contact with bases, hydrolysis will probably occur .
Flash point data for riddelline are not available; however, riddelline is probably combustible.
Riddelline is a natural alkaloid product
used a laboratory chemical and reference standard.
Riddelliine is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals and supporting data from studies on mechanisms of carcinogenesis.
UN1544 Alkaloids, solid, n.o.s. or Alkaloid salts,
solid, n.o.s. poisonous, Hazard Class: 6.1; Labels:
6.1-Poisonous materials, Technical Name Required.
Riddelline is sensitive to light, air, and
heat, causing oxidation; reacts slowly with atmospheric
oxygen. This chemical is probably combustible; dust mixed with air may be explosive. Incompatible with oxidizers
(chlorates, nitrates, peroxides, permanganates, perchlorates,
chlorine, bromine, fluorine, etc.); contact may cause fires
or explosions. Keep away from alkaline materials, strong
bases, strong acids, oxoacids, and epoxides. Contact with
alkalis and bases may cause hydrolysis.
It is inappropriate and possi-
bly dangerous to the environment to dispose of expired or
waste product such as lab chemicals by flushing them
down the toilet or discarding them to the trash. Larger
quantities shall carefully take into consideration applicable
EPA, and FDA regulations. If possible return the lab che-
micals to the manufacturer for proper disposal being careful
to properly label and securely package the material.
Alternatively, the waste lab chemicals shall be labeled,
securely packaged and transported by a state licensed medi-
cal waste contractor to dispose by burial in a licensed haz-
ardous or toxic waste landfill or incinerator.