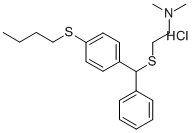
Covatine,Bailly,France,1958
p-Butylmercaptobenzhydryl chloride was boiled with thiourea in alcohol thereby yielding p-butylmercaptobenzhydrylisothiouronium chloride which was
then subjected to hydrolysis with dilute aqueous sodium hydroxide solution
whereupon p-butylmercaptobenzhydryl mercaptan was formed.
p-Butylmercaptobenzhydryl mercaptan (28.5 g) was added to a solution of
sodium (2.3 g) in absolute alcohol (75 ml), followed by the addition of a
solution of diethylaminoethyl chloride (13.6 g) in toluene (50 ml). The mixture
was boiled on a steam bath for 3 hours and the sodium chloride which
separated out was removed by filtration. The filtrate was concentrated to one-third of its volume and dissolved in ether. The ether solution was shaken with
2N hydrochloric acid (100 ml), and the resulting middle oily layer was
separated, dissolved in water and the resulting aqueous solution was washed
with ether, then treated with aqueous sodium hydroxide solution to precipitate
an oil. The latter was dissolved in ether, dried with anhydrous potassium
carbonate, filtered and then treated with anhydrous hydrogen chloride
whereupon the desired p-butylmercaptobenzhydryl 2-diethylaminoethyl sulfide
hydrochloride precipitated as a white, crystalline substance which was filtered
and dried in a desiccator. The melting point of the product was 124°C.