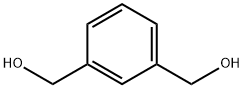
1,3-Benzenedimethanol was used in the synthesis of a series of cross-linked poly(orthocarbonate)s. It was also used in the synthesis of mixed-tethered system, required for the preparation of e-edge-[60]fullerenylmethanodihydropyrrole adduct.
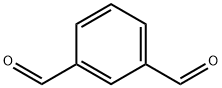
The general procedure for the synthesis of 1,3-benzenedimethanol from isophthalaldehyde was as follows: in a 25 mL round bottom flask equipped with a magnetic stirrer, an ethanol solution (5 mL) of isophthalaldehyde (1 mmol) was added. Subsequently, P(BVP)BH4 (100 mg) was added to the reaction system and the reaction was stirred at room temperature. The reaction process was monitored by thin layer chromatography (TLC). Upon completion of the reaction, the reaction mixture was filtered and washed sequentially with 1.0 M hydrochloric acid (2 × 10 mL) and ethanol (25 mL) to remove unreacted reagents. The filtrates were combined and concentrated under reduced pressure to give pure 1,3-benzenedimethanol in moderate to very high yields. For the few cases where the reaction is incomplete, the crude product needs to be purified by silica gel column chromatography using an appropriate eluent (see Scheme 1).
[1] Chemistry - A European Journal, 2013, vol. 19, # 24, p. 7701 - 7707
[2] Journal of Organic Chemistry, 2018, vol. 83, # 4, p. 2274 - 2281
[3] Comptes Rendus Chimie, 2013, vol. 16, # 8, p. 721 - 727
[4] Comptes Rendus Chimie, 2014, vol. 17, # 1, p. 23 - 29
[5] Indian Journal of Chemistry - Section A Inorganic, Physical, Theoretical and Analytical Chemistry, 2003, vol. 42, # 4, p. 744 - 750