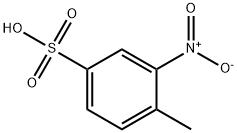
2-Nitrotoluene-4-sulfonic Acid, crystallizes from water as pale yellow hygroscopic needles of the dihydrate.
The total liquor of 2-Nitrotoluene-4-sulfonic Acid is hydrogenated in situ to give o-toluidine-4-sulfonic acid [618-03-1]. Isolated 2-nitrotoluene-4- sulfonic acid may be converted to 2-nitro-p-toluenesulfonyl chloride [54090-41-4], but the preferred process for [54090-41-4] is chlorosulfonation of 2-nitrotoluene. Reaction of [54090-41-4] with dimethylamine followed by reduction gives 3-amino-4-methyl-N,N-dimethylbenzenesulfonamide [6331-68-6] for use as an azoic diazo component.
2-Nitrotoluene is sulfonated by heating with 25 % oleum at 80 ℃ for 3 h, then quenching and liming to give a neutral solution of the sodium salt of 2-Nitrotoluene-4-sulfonic Acid.