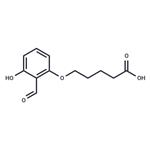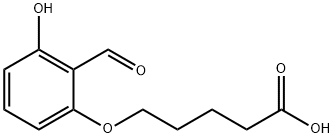
5-(2-formyl-3-hydroxyphenoxy)valeric acid
- Product Name5-(2-formyl-3-hydroxyphenoxy)valeric acid
- CAS77858-21-0
- MFC12H14O5
- MW238.24
- EINECS278-778-8
- MOL File77858-21-0.mol
Chemical Properties
| storage temp. | Store at -20°C |
| solubility | DMF: 16 mg/mL,DMSO: 10 mg/mL,Ethanol: Slightly soluble |
| form | A solid |
| color | Light yellow to light brown |
Usage And Synthesis
1st method of preparation of 5-(2-formyl-3-hydroxyphenoxy) pentanoic acid:
(A) 5-(2-Formyl-3-methoxyphenoxy)pentanoic acid:
2-Hydroxy-6-methoxybenzaldehyde (16.875 g, 0.111 M), ethyl 5- bromopentanoate (23.25 g, 17.6 ml, 0.111 M), anhydrous potassium carbonate (16.5 g), sodium iodide (0.675 g) and 95% ethanol (150 ml) were refluxed with stirring (16 hours). The cooled reaction mixture was filtered and the solid washed well with ethanol. The filtrate was evaporated to dryness and the residue partitioned between ether and water. The ethereal layer was separated and washed with 2 N sodium hydroxide solution, water, dried (sodium sulfate) and evaporated. The residue was dissolved in 95% ethanol (300 ml) and 0.66 N sodium hydroxide solution (450 ml) and stirred at ambient temperature (4 hours). The reaction mixture was evaporated to half volume and diluted with water. The mixture was extracted once with ether and the aqueous layer acidified with concentrated hydrochloric acid with cooling. The crystalline solid formed was filtered off and washed well with water. Recrystallisation from ethyl acetate-petrol gave 5-(2-formyl-3- methoxyphenoxy)pentanoic acid, melting point 99-101°C.
(B) 5-(2-Formyl-3-hydroxyphenoxy)pentanoic acid: 5-(2-Formyl-3-methoxyphenoxy)pentanoic acid (504 mg, 0.002 M) was dissolved in anhydrous dichloromethane (20 ml) and cooled to -70°C. A solution of boron trichloride in anhydrous dichloromethane (0.25 g/ml, 3.76 ml) was added dropwise over 10 min and the mixture stirred at -70°C (15 min). The reaction mixture was allowed to reach ambient temperature and stirred at that temperature (1.25 hours). After cooling to 10°C, 10% sodium acetate solution (15 ml) was added dropwise with stirring so that the temperature did not rise above 15°C. The resulting mixture was diluted with ethyl acetate (50 ml) and filtered. The filtrate was transferred to a separating funnel and the aqueous layer separated. The organic phase was extracted with 10% sodium carbonate solution (2 x 50 ml), the combined extracts acidified with concentrated hydrochloric acid and extracted with ethyl acetate. The combined extracts were washed with water, dried (sodium sulfate) and evaporated to give a crystalline solid. This solid was dissolved in the minimum of chloroform-methanol (95:5) and passed through a pad of Kieselgel G. Evaporation of the filtrate and recrystallization from benzene-petrol gave 5-(2- formyl-3-hydroxyphenoxy)pentanoic acid, melting point 97-99°C.
2nd method of preparation of 5-(2-formyl-3-hydroxyphenoxy)pentanoic acid:
(A) Ethyl 5-(2-formyl-3-benzyloxypheoxy)pentanoate:
A mixture of 2-hydroxy-6-benzyloxybenzaldehyde (3.0 g, 0.013 M), ethyl 5- bromopentanoate (2.75 g, 0.013 M), anhydrous potassium carbonate (2.16 g, 0.0156 M), sodium iodide (0.195 g) and dry dimethylformamide (15 ml) were stirred at 60-80°C for 3 hours and then left to stir at room temperature overnight. The mixture was then poured into water (50 ml) and the product extracted with ether (2 x 80 ml) and the combined extracts washed with 10% aqueous sodium hydroxide (2 x 20 ml) and then with water to neutrality, dried, and evaporated to give ethyl 5-(2-formyl-3- benzyloxyphenoxy)pentanoate, (4.0 g, 86%) as a pale yellow oil.
(B) 5-(2-Formyl-3-benzyloxyphenoxy)pentanoic acid:
A mixture of ethyl 5-(2-formyl-3-benzyloxyphenoxy)pentanoate (3.61 g, 0.01 M), potassium hydroxide (1.19 g, 0.021 M) and ethanol (40 ml) were stirred at 50°-60°C for 5 hours. The ethanol was then removed in vacuum, the residue dissolved in water (50 ml) and the solution extracted with ether (2 x 80 ml). The aqueous layer was then acidified by the addition of 2 N aqueous hydrochloric acid and the product extracted with ether, and the combined extracts washed with water to neutrality, dried, and concentrated in vacuum to give 5-(2-formyl-3-benzyloxyphenoxy)pentanoic acid, 3.0 g, 91% as a yellow oil which crystallized on standing. The crude solid was crystallized from benzene/petroleum ether to give pale cream crystals, melting point 110°C.
(C) 5-(2-Formyl-3-hydroxyphenoxy)pentanoic acid:
A solution of 5-(2-formyl-3-benzyloxyphenoxy)pentanoic acid (1.0 g, 0.003 M) in ethanol containing 5% palladium on charcoal catalyst (0.61 g) was hydrogenated at atmospheric pressure. After 20 min the reaction was complete and the catalyst was filtered off and the ethanol removed in vacuum to give 5-(2-formyl-3-hydroxyphenoxy)pentanoic acid, m.p. 94°C.
3rd method of preparation of 5-(2-formyl-3-hydroxyphenoxy)pentanoic acid:
(A) Ethyl 5-(2-formyl-3-methoxyphenoxy)pentanoate:
2-Hydroxy-6-methoxybenzaldehyde (26.0 g, 0.17 M), ethyl 5- bromopentanoate (27.1 ml, 0.17 M), anhydrous potassium carbonate (25.4 g), sodium iodide (1.04 g) and ethanol (230 ml) were refluxed with stirring for 16 hours. The cooled reaction mixture was filtered and the solid washed well with ethanol. The filtrate was evaporated to dryness and the residue partitioned between ether (200 ml) and water (200 ml). The organic layer was separated and washed with 2 N sodium hydroxide solution, water, brine, dried (magnesium sulfate) and evaporated to yield ethyl 5-(2-formyl-3- methoxyphenoxy)pentanoate 32.97 g, 67% yield, as a pale yellow oil that solidified on standing in the refrigerator.
(B) 5-(2-Formyl-3-hydroxyphenoxy)pentanoic acid:
10 ml of a solution of iodine (40.3 g, 0.157 M) in ether (sodium dry, 500 ml) was added to a stirred mixture of magnesium metal (15.4 g, 0.636 GATOM) and ether (50 ml). When the reaction had commenced the remainder of the iodine solution was added dropwise at such a rate as to cause gentle refluxing. After the addition was complete the reaction mixture was heated to reflux until a colorless solution was obtained (1/2 hour). The cooled reaction mixture was filtered and the unreacted magnesium metal was washed with ether (100 ml). The colorless solution of magnesium iodide thus obtained was added dropwise to a solution of ethyl 5-(2-formyl-3- methoxyphenoxy)pentanoate (30.0 g, 0.106 M) in tetrahydrofuran (dried over molecular sieve, 300 ml) at such a rate as to cause gentle refluxing. A fine yellow precipitate dropped out of solution. The mixture was brought to reflux with stirring for 5 hours. The cooled reaction mixture was poured into 10% hydrochloric acid (400 ml). The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined organic phases, containing ethyl 5-(2-formyl-3-hydroxyphenoxy)pentanoate, were washed with water and then extracted into 2 N sodium hydroxide solution. The combined aqueous extracts were acidified with concentrated hydrochloric acid with ice- cooling. The precipitate was filtered, washed with water, sucked dry and then quickly washed with a petrol/ethanol mixture (6:1, 60 ml) to remove some of the color. The crude product was dried in a desiccator over phosphorus pentoxide to give a dark-peach colored solid which was then dissolved in ethyl acetate (250 ml); aluminum oxide (neutral, 10 g) and charcoal (5.0 g) were added and the mixture stirred vigorously for 1/2 hour and then filtered to give a pale-yellow solution. The solvent was removed in vacuum to give 5-(2- formyl-3-hydroxyphenoxy)pentanoic acid, melting point 98-99°C (from ethyl acetate/petrol).
(A) 5-(2-Formyl-3-methoxyphenoxy)pentanoic acid:
2-Hydroxy-6-methoxybenzaldehyde (16.875 g, 0.111 M), ethyl 5- bromopentanoate (23.25 g, 17.6 ml, 0.111 M), anhydrous potassium carbonate (16.5 g), sodium iodide (0.675 g) and 95% ethanol (150 ml) were refluxed with stirring (16 hours). The cooled reaction mixture was filtered and the solid washed well with ethanol. The filtrate was evaporated to dryness and the residue partitioned between ether and water. The ethereal layer was separated and washed with 2 N sodium hydroxide solution, water, dried (sodium sulfate) and evaporated. The residue was dissolved in 95% ethanol (300 ml) and 0.66 N sodium hydroxide solution (450 ml) and stirred at ambient temperature (4 hours). The reaction mixture was evaporated to half volume and diluted with water. The mixture was extracted once with ether and the aqueous layer acidified with concentrated hydrochloric acid with cooling. The crystalline solid formed was filtered off and washed well with water. Recrystallisation from ethyl acetate-petrol gave 5-(2-formyl-3- methoxyphenoxy)pentanoic acid, melting point 99-101°C.
(B) 5-(2-Formyl-3-hydroxyphenoxy)pentanoic acid: 5-(2-Formyl-3-methoxyphenoxy)pentanoic acid (504 mg, 0.002 M) was dissolved in anhydrous dichloromethane (20 ml) and cooled to -70°C. A solution of boron trichloride in anhydrous dichloromethane (0.25 g/ml, 3.76 ml) was added dropwise over 10 min and the mixture stirred at -70°C (15 min). The reaction mixture was allowed to reach ambient temperature and stirred at that temperature (1.25 hours). After cooling to 10°C, 10% sodium acetate solution (15 ml) was added dropwise with stirring so that the temperature did not rise above 15°C. The resulting mixture was diluted with ethyl acetate (50 ml) and filtered. The filtrate was transferred to a separating funnel and the aqueous layer separated. The organic phase was extracted with 10% sodium carbonate solution (2 x 50 ml), the combined extracts acidified with concentrated hydrochloric acid and extracted with ethyl acetate. The combined extracts were washed with water, dried (sodium sulfate) and evaporated to give a crystalline solid. This solid was dissolved in the minimum of chloroform-methanol (95:5) and passed through a pad of Kieselgel G. Evaporation of the filtrate and recrystallization from benzene-petrol gave 5-(2- formyl-3-hydroxyphenoxy)pentanoic acid, melting point 97-99°C.
2nd method of preparation of 5-(2-formyl-3-hydroxyphenoxy)pentanoic acid:
(A) Ethyl 5-(2-formyl-3-benzyloxypheoxy)pentanoate:
A mixture of 2-hydroxy-6-benzyloxybenzaldehyde (3.0 g, 0.013 M), ethyl 5- bromopentanoate (2.75 g, 0.013 M), anhydrous potassium carbonate (2.16 g, 0.0156 M), sodium iodide (0.195 g) and dry dimethylformamide (15 ml) were stirred at 60-80°C for 3 hours and then left to stir at room temperature overnight. The mixture was then poured into water (50 ml) and the product extracted with ether (2 x 80 ml) and the combined extracts washed with 10% aqueous sodium hydroxide (2 x 20 ml) and then with water to neutrality, dried, and evaporated to give ethyl 5-(2-formyl-3- benzyloxyphenoxy)pentanoate, (4.0 g, 86%) as a pale yellow oil.
(B) 5-(2-Formyl-3-benzyloxyphenoxy)pentanoic acid:
A mixture of ethyl 5-(2-formyl-3-benzyloxyphenoxy)pentanoate (3.61 g, 0.01 M), potassium hydroxide (1.19 g, 0.021 M) and ethanol (40 ml) were stirred at 50°-60°C for 5 hours. The ethanol was then removed in vacuum, the residue dissolved in water (50 ml) and the solution extracted with ether (2 x 80 ml). The aqueous layer was then acidified by the addition of 2 N aqueous hydrochloric acid and the product extracted with ether, and the combined extracts washed with water to neutrality, dried, and concentrated in vacuum to give 5-(2-formyl-3-benzyloxyphenoxy)pentanoic acid, 3.0 g, 91% as a yellow oil which crystallized on standing. The crude solid was crystallized from benzene/petroleum ether to give pale cream crystals, melting point 110°C.
(C) 5-(2-Formyl-3-hydroxyphenoxy)pentanoic acid:
A solution of 5-(2-formyl-3-benzyloxyphenoxy)pentanoic acid (1.0 g, 0.003 M) in ethanol containing 5% palladium on charcoal catalyst (0.61 g) was hydrogenated at atmospheric pressure. After 20 min the reaction was complete and the catalyst was filtered off and the ethanol removed in vacuum to give 5-(2-formyl-3-hydroxyphenoxy)pentanoic acid, m.p. 94°C.
3rd method of preparation of 5-(2-formyl-3-hydroxyphenoxy)pentanoic acid:
(A) Ethyl 5-(2-formyl-3-methoxyphenoxy)pentanoate:
2-Hydroxy-6-methoxybenzaldehyde (26.0 g, 0.17 M), ethyl 5- bromopentanoate (27.1 ml, 0.17 M), anhydrous potassium carbonate (25.4 g), sodium iodide (1.04 g) and ethanol (230 ml) were refluxed with stirring for 16 hours. The cooled reaction mixture was filtered and the solid washed well with ethanol. The filtrate was evaporated to dryness and the residue partitioned between ether (200 ml) and water (200 ml). The organic layer was separated and washed with 2 N sodium hydroxide solution, water, brine, dried (magnesium sulfate) and evaporated to yield ethyl 5-(2-formyl-3- methoxyphenoxy)pentanoate 32.97 g, 67% yield, as a pale yellow oil that solidified on standing in the refrigerator.
(B) 5-(2-Formyl-3-hydroxyphenoxy)pentanoic acid:
10 ml of a solution of iodine (40.3 g, 0.157 M) in ether (sodium dry, 500 ml) was added to a stirred mixture of magnesium metal (15.4 g, 0.636 GATOM) and ether (50 ml). When the reaction had commenced the remainder of the iodine solution was added dropwise at such a rate as to cause gentle refluxing. After the addition was complete the reaction mixture was heated to reflux until a colorless solution was obtained (1/2 hour). The cooled reaction mixture was filtered and the unreacted magnesium metal was washed with ether (100 ml). The colorless solution of magnesium iodide thus obtained was added dropwise to a solution of ethyl 5-(2-formyl-3- methoxyphenoxy)pentanoate (30.0 g, 0.106 M) in tetrahydrofuran (dried over molecular sieve, 300 ml) at such a rate as to cause gentle refluxing. A fine yellow precipitate dropped out of solution. The mixture was brought to reflux with stirring for 5 hours. The cooled reaction mixture was poured into 10% hydrochloric acid (400 ml). The organic layer was separated and the aqueous layer was extracted with ethyl acetate. The combined organic phases, containing ethyl 5-(2-formyl-3-hydroxyphenoxy)pentanoate, were washed with water and then extracted into 2 N sodium hydroxide solution. The combined aqueous extracts were acidified with concentrated hydrochloric acid with ice- cooling. The precipitate was filtered, washed with water, sucked dry and then quickly washed with a petrol/ethanol mixture (6:1, 60 ml) to remove some of the color. The crude product was dried in a desiccator over phosphorus pentoxide to give a dark-peach colored solid which was then dissolved in ethyl acetate (250 ml); aluminum oxide (neutral, 10 g) and charcoal (5.0 g) were added and the mixture stirred vigorously for 1/2 hour and then filtered to give a pale-yellow solution. The solvent was removed in vacuum to give 5-(2- formyl-3-hydroxyphenoxy)pentanoic acid, melting point 98-99°C (from ethyl acetate/petrol).
Preparation Products And Raw materials
5-(2-formyl-3-hydroxyphenoxy)valeric acid manufacturers
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine