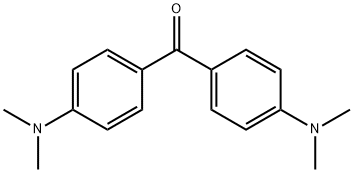
Michler's ketone is a blue powder or white togreen-colored leaflet material. Molecular weight = 268.37;Boiling point ≥360℃ (decomposition); Freezing/Meltingpoint= 172-176*C; Flash point = 220-C; Autoignitiontemperature = 480PC. Hazard Identification (based onNFPA-704 M Rating System): Health 3, Flammability 1,Reactivity 0. Insoluble in water.
Michler’s ketone is a is a blue powder or
white to green-colored leaflet material.
white to light greenish crystals
MK can be used as an additive that acts as a photoinitiator in the preparation of dyes. It can also be used as a precursor material in the synthesis of 4,4′-bis{N,N,dimethyl, N (2-ethoxy carbonyl-1-propenyl) ammonium hexafluoro antimonate}benzophenone (MKEA).
In manufacture of dyes and pigments.
ChEBI: 4,4'-Bis(dimethylamino)benzophenone is a member of benzophenones.
White to greenish crystalline leaflets or blue powder.
4,4'-Bis(dimethylamino)benzophenone is incompatible with strong oxidizing agents and strong reducing agents .
Literature sources indicate that 4,4'-Bis(dimethylamino)benzophenone is combustible.
Confirmed human
carcinogen with experimental carcinogenic
and neoplastigenic data. A poison by
ingestion. Mutation data reported. A
flammable liquid. When heated to
decomposition it emits toxic fumes of NOx.
Mutagen. Animal Carcinogen.
Michler’s ketone is a dye intermediate and derivative of
dimethylaniline. It is also used in antifreeze formulations,
cosmetics, cleaning compounds; heat transfer fluids;
as a chemical intermediate in the synthesis of at least 13
dyes and pigments, especially auramine derivatives.
Skin Contacr52: Flood all areas of body thathave contacted the substance with water.Do not wait tofemove contaminated clothing; do it under the water stream.Use soapto help assure removal. Isolate contaminatedclothing when removed to prevent contact by others. EyeContact: Remove any contact Tenses at once. Immediatelyflush eyes well with copious quantities of water or normalsaline for at least 20- 30 min. Seek medical attention.Imhalation: Leave contaminated area immediately; breathefresh air. Proper respiratory protection must be supplied toany rescuers. If coughing, difficult breathing. or any othersymptoms develop, seek medical attention at once, even isymptoms develop many hours after exposure. Ingestion: IFunconscious or convulsing, do not induce vomiting or giveanything by mouth. Assure that victim's airway is open andlay him on his side with his head lower than his body andfransport at once to a medical facility. If conscious and noCconvutsing, give a glass of water to dilute the substance. Imedical advice is not readily available, do not inducevomiting, and rush the victim to the nearest medicalfacility.
Michler’s ketone is reasonably anticipated to be a human cagen based on sufficient evidence of carcinogenicity from stud
rcinoies in experimental animals.
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in a refrigerator or a cool, dry place awayfrom peroxides, aldehydes, strong acids. Where possible,automatically pump liquid from drums or other storage containers to process containers. A regulated, marked areashould be established where this chemical is handled, used,or stored in compliance with OSHA Standard 1910.1045.
UN3143 Dyes, solid, toxic, n.o.s. or Dye intermediates,
solid, toxic, n.o.s., Hazard Class: 6.1; Labels:
6.1-Poison Inhalation Hazard. UN2811 Toxic solids,
organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous
materials, Technical Name Required. UN1602 Dyes, liquid,
toxic, n.o.s or Dye intermediates, liquid, toxic, n.o.s.,
Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Dissolve the ketone in dilute HCl, filter and precipitate it by adding ammonia (to remove water-insoluble impurities such as benzophenone). Then crystallise it from EtOH or pet ether. [Suppan J Chem Soc, Faraday Trans1 71 539 1975.] It is also purified by dissolving in *benzene, then washing with water until the aqueous phase is colourless. The *benzene is evaporated off, and the residue is recrystallised three times from *benzene and EtOH [Hoshino & Kogure J Phys Chem 72 417 1988]. [Beilstein 14 IV 255.]
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates,
chlorine, bromine, fluorine, etc.); contact may cause fires
or explosions. Keep away from aldehydes, alkaline
materials, strong acids, strong bases, strong reducing
agents such as hydrideds and active metals. Contact
with hydrogen peroxide may form heat- and shock- sensitive
explosives.
Do not discharge into drains
or sewers. Consult with environmental regulatory agencies
for guidance on acceptable disposal practices. If allowed,
Incineration with effluent gas scrubbing is recommended.
Containers must be disposed of properly by following
package label directions or by contacting your local or
federal environmental control agency, or by contacting
your regional EPA office.