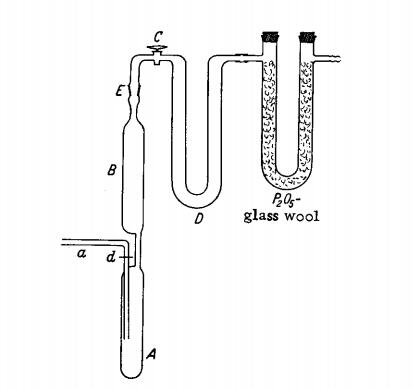
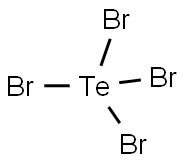
Pure Te (5 g.) is introduced through adapter a into the elongated reaction flask A of the apparatus shown in Fig.1. Adapter a is connected to a N2 purification train which furnishes either pure dry N2 or N2 containing bromine vapor. For the latter, the gas stream may be passed through a wash bottle containing dry Br2 , followed by a U tube containing P2O5-glass wool. First, the apparatus in purged with pure N2. Then A is cooled with ice water and the N2 -Br2 mixture is introduced. A portion of the Br2 condenses on the Te and reacts quietly with it, while the remainder is retained in the empty U tube D, cooled with an ice-salt mixture. The difference between the weight loss of the Br2 wash bottle and the weight of the condensate in D indicates the amount of bromine remaining in A. When this becomes about twice the amount needed for quantitative conversion to TeBr4, the gas stream is interrupted, stopcock C is closed, and the product slurry is allowed to stand at room temperature for several hours (better overnight) in order to complete the reaction. Then C is reopened and the excess bromine is purged by a stream of N2 while A is simultaneously heated to 50℃. To purify the product, it is sublimed in vacuum. Gas inlet tube a is sealed off at d, the whole apparatus is tilted to a horizontal position, and ground glass joint E is connected to an aspirator through a P2O5 drying tube. During evacuation, A is heated to sublimation temperature (about 350℃) with an electric furnace. Any black condensate which may separate in B at 200℃ is vaporized by heating with a burner. The subsequently deposited yellow to orange-red powder is quite pure TeBr4; if necessary, this can be further purified by an analogous sublimation in high vacuum.