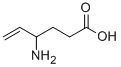
Vigabatrin is a second- generation anti- epileptic drug (AED), known under the proprietary brand name of Sabril® (Sanofi, Paris) in the UK and USA.
MHRA/ CHM advice to minimize risk when switching patients with epilepsy between different manufacturers’ products (including generic products):
- It is usually unnecessary to ensure that patients are maintained on a specific manufacturer’s product unless there are specific concerns, such as patient anxiety and risk of confusion/ dosing error.
Epilepsy: adjunctive therapy of focal seizures with or without secondary generalization not satisfactorily controlled with other AEDs.
Recommendations summarized from NICE (2012)
- Seizure types: on referral to tertiary care (focal seizures), contraindicated (generalized tonic- clonic seizures, tonic/ atonic seizures, absence seizures, myoclonic seizures).
- Epilepsy types: on referral to tertiary care (benign epilepsy with centrotemporal spikes, panayiotopoulos syndrome, late- onset childhood occipital epilepsy), contraindicated (absence syndromes, idiopathic generalized epilepsy, juvenile myoclonic epilepsy, Dravet syndrome, Lennox– Gastaut syndrome).
Epilepsy
Adjunctive therapy: 1000 mg daily divided into 1 or 2 doses for 7 days, then increased by 500 mg 7 days; usual maintenance 2000– 3000 mg daily (max. 3000 mg daily).
No direct correlation exists between the plasma concentration and the efficacy
of vigabatrin. The duration of the effect of the medicinal product is dependent on
the rate of GABA transaminase re- synthesis, rather than the concentration of the
drug in the plasma. The routine measurement of plasma levels in clinical practice
is therefore, unnecessary.
- Patients with a history of behavioural problems, depression, psychosis.
- Elderly patients.
- Patients with visual field defects (contraindication).
Vigabatrin can be associated with adverse effects at the level the nervous system
and other systems.
With AEDs
As vigabatrin is neither metabolized, nor protein bound and is not an inducer of hepatic cytochrome P450 drug metabolizing- enzymes, there are no significant interactions with other drugs. Controlled clinical studies have shown a gradual reduction of 16– 33% in the plasma concentration of phenytoin (unlikely to be of therapeutic significance).
With other drugs
Nil.
With alcohol/food
There are no known specific interactions between alcohol and vigabatrin and there are no specific foods that must be excluded from diet when taking vigabatrin (food administration does not alter the extent of vigabatrin absorption).
Hepatic impairment
No dose adjustment is required for patients with hepatic impairment.
Renal impairment
Consider reducing maintenance dose or frequency of administration.
Pregnancy
- Based on data on pregnancies exposed to vigabatrin, no definite conclusion can be drawn as to whether vigabatrin produces an increased risk of malformation when taken during pregnancy because of limited data and the administration of concomitant AEDs.
- Vigabatrin should not be used during pregnancy unless it is required based on the clinical condition of the patient. In such cases, the dose of vigabatrin should be monitored carefully during pregnancy and after birth, and adjustments made on a clinical basis.
- Vigabatrin is excreted in human milk: since there is insufficient information on the effects of vigabatrin in newborns/ infants, the possibility of avoiding breast- feeding should be considered.
Behavioural and cognitive effects in patients with epilepsy
Patients treated with vigabatrin often report behavioural adverse effects (most frequently depression, psychosis, and irritability). Risk factors for developing adverse psychiatric effects during vigabatrin therapy include high starting and maintenance doses, past psychiatric history and epilepsy severity. Vigabatrin is characterized by a positive cognitive profile, with rare reports of memory, attention, and language problems.
Vigabatrin has no approved indications in psychiatry. There is weak evidence for usefulness in the treatment of anxiety disorders and addictions.
Vigabatrin, the gamma-vinyl derivative of GABA, is a new anticonvulsant reportedly
effective in the treatment of intractable seizures unresponsive to currently available
therapy. Mechanistically vigabatrin is a potent irreversible GABA aminotransferase
inhibitor which modifies the enzyme's active-site by Michael addition. Other potential
indications have been suggested for vigabatrin, including depression and schizophrenia.
White or almost white powder.
Merrell Dow (United Kingdom)
Vigabatrin is a selective GABA transaminase inhibitor.
ChEBI: A gamma-amino acid having a gamma-vinyl GABA structure. It is an irreversible inhibitor of gamma-aminobutyric 664 acid transaminase
Step A: 4-Formyloxy-3-hydroxy-1-butene
A solution of erythritol (50 g, 0.5 mole) in aqueous formic acid (150 g, 75%)
was heated above 100°C, 12 hours, then water and formic acid were distilled
off and the reaction mixture was heated above 200°C with a Bunsen burner.
The product was collected by distillation (b.p. 230°C, 30 g) and should be
rectified (b.p. 90°C, 15 mm).
Step B: Ethyl 6-formyloxy-4-hexanoate
A solution of 4-formyloxy-3-hydroxy-1-butene (1.06 g, 10 mmol) and
propionic acid (1 drop) in triethylorthoacetate (6 g, 40 mmol) was heated at
140°C under conditions for distillative removal of ethanol. After 2 hours, the
excess of ethylorthoacetate was removed by distillation in vacuo. The residue
was hydrolysed with water and extracted with AcOEt. The product was purified
by flash chromatography on SiO 2 (eluant AcOEt:hexane, 2:8) (1 g, 60%) but
distillative purification is preferred when larger quantities are involved.
Step C: Ethyl 6-hydroxy-4-hexanoate
A solution of 6-formyloxy-6-hexanoate (0.9 g, 5 mmol) in absolute EtOH (10
mL) containing few drops of a saturated solution of alcoholic HCl gas was left
2 hours at 20°C. The solvent was removed in vacuo and the residue was used
for the next step without further purification (0.7 g, quantitative). This
compound was found to be partially decomposed by flash chromatography on
SiO 2 .
Step D: Ethyl 4-trichloroacetamido-5-hexanoate
Sodium hydride (0.03 g of a 50% dispersion in oil, 0.5 mmol, was added to a
solution of ethyl 6-hydroxy-4-hexanoate (0.7 g, 5 mmol) and
trichloroacetonitrile (0.6 g, 5 mmol) in anhydrous ether (50 mL) under
N2at0°C. After 1hour, ethanol (0.5 mmol) was added and the solvent was
removed in vacuo. The formation of the imidate was controlled by NMR (NH,
about.8.5 ppm). A solution of the crude imidate in xylene (30 mL) was heated
at reflux 48 hours. Then the solvent was removed in vacuo and the residue
was purified by flash chromatography on SiO 2 (eluant AcOEt:hexane, 2:8) to give the title product (1.1 g, about 70%). A sample was distilled for analysis
(b.p. 150°C, 0.5 mm Hg).
Step E: 4-Amino-5-hexenoic acid
A suspension of ethyl 4-trichloroacetoamido-5-hexanoate (0.3 g, 1 mmol) in 6
N HCl (10 mL) was heated under reflux 6 hours. Then the mixture was
concentrated in vacuo, diluted with water (10 mL), washed twice with AcOEt,
and dried in uacuo to give the title product (0.18 g, 100%). NMR, TLC
(NH 4 OH:EtOH, 3:7) are identical with those of an authentic sample of 4-
amino-5-hexenoic acid
Sabril (Hoechst Marion Roussel);Sobril tab 25 mg.
World Health Organization (WHO)
Vigabatrin, an irreversible inhibitor of GABA-transaminase was
introduced in 1989 as a anticonvulsant for management of epilepsy unresponsive
to other antiepilepsy agents. In 1991 it was refused registration in Norway because
it induced toxic changes, including microvacuolation in the brain of two animal
species, at doses that are close to therapeutic dosage levels in man. It is still
marketed in Sweden and the United Kingdom.
Vigabatrin (Sabril) is a relatively specific irreversible inhibitor
of GABA-transaminase (GABA-T), the major
enzyme responsible for the metabolism of GABA in the
mammalian CNS. As a result of inhibition of GABA-T,
there is an increase in the concentration of GABA in the
brain and consequently an increase in inhibitory neurotransmission.
Vigabatrin is well absorbed orally and is
distributed to all body systems.The major route of elimination
for vigabatrin is renal excretion of the parent compound;
no metabolites have been identified in humans.
At present, the primary indication for vigabatrin is
in the treatment of patients with partial seizures, but it
appears to be an effective and generally well tolerated
antiepileptic medication for other seizure types as well.
It should not be used in patients with absence epilepsy
or with myoclonic seizures. Vigabatrin is not approved
as an AED in the United States, although it is approved
in many other countries.
Vigabatrin, a 4-vinyl analog of GABA, produces its pharmacologicalaction by irreversibly blocking GABA catabolismcatalyzed by GABA-T as discussed earlier. It is marketedin Europe and Canada as an adjunctive treatment ofpatients with partial seizures, but it has yet to gain FDA approvalin the United States even after extensive clinical trials.The main concern with this drug is its ability to causea reversible visual field defect associated with retinal functionin the eyes.
Selective GABA-T inhibitor. Anticonvulsant.
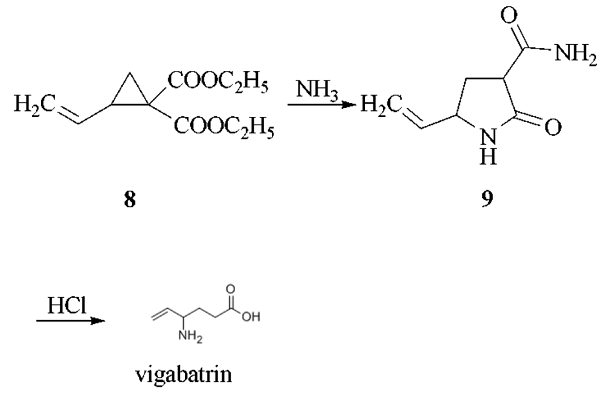
The reaction of 1,4-dichloro-2- butene with diethyl malonate in the presence of sodium ethoxide as catalyst in refluxing ethanol gives 1,1-bis(ethoxycarbonyl)-2-vinylcyclopropane (8), which by reaction with gaseous ammonia in DMF is converted into 3-carboxamido- 5-vinyl-2-pyrrolidone (9). This compound is treated with HCl in refluxing acetic acid to yield vigabatrin.
Potentially hazardous interactions with other drugs
Antidepressants: anticonvulsant effect antagonised,
convulsive threshold lowered; avoid with St John’s
wort.
Antiepileptics: concentration of phenytoin reduced.
Antimalarials: mefloquine antagonises
anticonvulsant effect.
Antipsychotics: anticonvulsant effect antagonised.
Orlistat: increased risk of convulsions.
Vigabatrin is not significantly metabolised. About
60-80
% of an oral dose is excreted in urine as unchanged
drug.