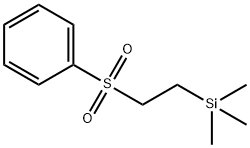
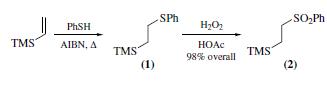
(2-Phenylsulfonylethyl)trimethylsilane is widely used as reagent for the synthesis of mono- and 1,1-disubstituted alkenes via sulfone metalation, alkylation, and fluoride-induced elimination.
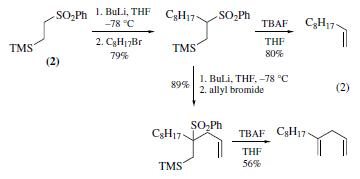
A sequence involving metalation, alkylation, and fluoride-induced elimination of benzenesulfinate allows the conversion of (2) to a terminal alkene. An analogous sequence involving a double alkylation of (2) provides a 1,1-disubstituted alkene (eq 2). The lithio derivative of (2) has also been used to prepare cyclopropylidene derivatives, homoallylic alcohols, and allyl silanes via the Julia alkenation.