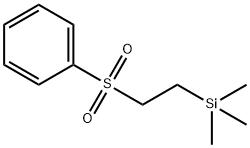
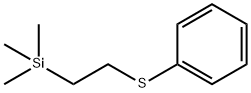
Uses
(2-Phenylsulfonylethyl)trimethylsilane is widely used as reagent for the synthesis of mono- and 1,1-disubstituted alkenes via sulfone metalation, alkylation, and fluoride-induced elimination.
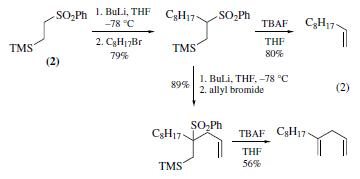
A sequence involving metalation, alkylation, and fluoride-induced elimination of benzenesulfinate allows the conversion of (2) to a terminal alkene. An analogous sequence involving a double alkylation of (2) provides a 1,1-disubstituted alkene (eq 2). The lithio derivative of (2) has also been used to prepare cyclopropylidene derivatives, homoallylic alcohols, and allyl silanes via the Julia alkenation.
Preparation
(2-phenylsulfonylethyl)trimethylsilane
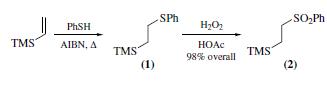
(2) is prepared by radical addition of thiophenol to
vinyltrimethylsilane to give (2-phenylthioethyl)trimethylsilane
(1), which is then oxidized with hydrogen peroxide).
Purification Methods
Dissolve it in Et2O, wash it with saturated HCO 3 followed by saturated NaCl, H2O and dried (MgSO4). Evaporation leaves residual crystals with m 52o. [Hsiao & Shechter Tetrahedron Lett 23 1963 1982, Bortolini et al. J Org Chem 53 2688 1985.]