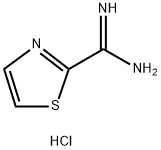
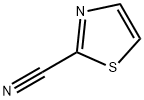
General procedure for the synthesis of 2-thiazolecarboxamidine hydrochloride from 2-cyanothiazole: Methanol (12 L) was added to a SQL reactor, followed by 2-cyanothiazole (2200 g, 20.00 mol, 1 eq.) and sodium methanolate (54 g, 1 mol, 0.05 eq.). The mixture was stirred at 10°C for 0.5 hours. The course of the reaction was monitored by TLC (petroleum ether: ethyl acetate = 2:1) to confirm the disappearance of the feedstock and to detect the formation of intermediates. Next, ammonium chloride (1296 g, 24.00 mol, 1.2 eq.) was added and the mixture was stirred at 65 °C for 16 hours. The disappearance of the intermediate was again confirmed by TLC (petroleum ether: ethyl acetate = 2:1) indicating completion of the reaction. The reaction mixture was filtered to remove solid ammonium chloride (210 g) and the filtrate was dried to give the crude product. The crude product was slurried with ethyl acetate (20 L) for 2 h and then filtered to give 2-thiazolecarboxamidine hydrochloride (3200 g) with 98% purity. The effective content was 95% as determined by quantitative NMR. The isolated yield was 93%.1H NMR (400 MHz, DMSO-d6) δ 8.26 (d, J = 2.76 Hz, 1H), 8.38 (d, J = 3.01 Hz, 1H), 9.81 (br.s., 1H).LCMS: m/z 127.9 [M + H]+.