Aceclofenac is a non-steroidal anti-inflammatory drug (NSAIDs) that is commonly prescribed for people with painful rheumatic conditions such as rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis.
The drug should not be given to children, breastfeeding mothers, and people with porphyria. Pregnant women should not also be given aceclofenac as they risk developing patent ductus arteriosus in the neonate.
Aceclofenac acts by inhibiting the effect of natural substances known as cyclooxygenase (COX) enzymes. Notably, these enzymes are responsible for making other chemicals in the body, namely prostaglandins. The prostaglandins are normally produced at sites of damages or injury cause inflammation and pain. By blocking the influence of COX enzymes, production of prostaglandins is minimized, meaning that the swelling and pain is eased.
Before taking aceclofenac, it is essential to tell the doctor if one has ever had an allergic reaction to any other NSAID, for instance, diclofenac, aspirin, indomethacin, and naproxen; whether one has allergic disorders such as asthma. It is important to alert the doctor if an individual has a heart condition or problem with circulation or blood vessels. Moreover, inform the physician if one has connective tissue disorder, for instance, lupus erythematosus. One should not take the drug if he/she has high blood pressure or has blood-clotting problems.
Aceclofenac is a nonsteroidal antiinflammatory agent with analgesic and antipyretic
properties. It is reported to be useful in the treatment of osteoarthritis, rheumatoid
arthritis and pain associated with minor surgical procedures. Compared to ketoprofen
in the treatment of rheumatoid arthritis, aceclofenac is substantially faster acting.
Prodes (Prodesfarma) (Spain)
Aceclofenac is a non-steroidal, anti-inflammatory drug (NSAID) with potent inhibitory activity in several models of inflammation. It is used for the treatment of osteoarthritis and rheumatoid arthritis. It is a Biopharmaceutics classification system class II (BCS class I) drug which has an intermediate half-life of 3-4h and undergoes substantial first pass metabolism. aceclofenac is available either in oral form (tablet) or in topical form (gel).
Labeled Aceclofenac, intended for use as an internal standard for the quantification of Aceclofenac by GC- or LC-mass spectrometry.
ChEBI: Aceclofenac is a monocarboxylic acid that is the carboxymethyl ester of diclofenac. A non-steroidal anti-inflammatory drug related to diclofenac, it is used in the management of osteoarthritis, rheumatoid arthritis, and ankylosing spondylitis. It has a role as an EC 1.14.99.1 (prostaglandin-endoperoxide synthase) inhibitor, a non-steroidal anti-inflammatory drug and a non-narcotic analgesic. It is a monocarboxylic acid, a carboxylic ester, a secondary amino compound, an amino acid and a dichlorobenzene. It derives from a diclofenac.
Analgesic, Antiinflammatory
Non-steroidal, anti-inflammatory drug (NSAID), with selectivity for COX-2 over COX-1.
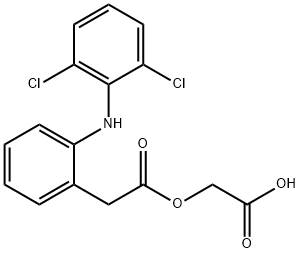
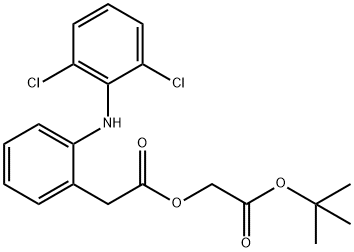
General procedure for the synthesis of 2-((2,6-dichlorophenyl)amino)phenylacetoxyacetic acid from 2-(tert-butoxy)-2-oxoethyl 2-(2-((2,6-dichlorophenyl)amino)phenyl)acetic acid: 500g of 2-(tert-butoxy)-2-oxoethyl 2-(2-((2,6-dichlorophenyl)amino)phenyl)acetic acid was added to a 2L three-neck flask. 500 ml of acetic acid, 25 ml of concentrated hydrochloric acid solution (mass fraction: 37%), 500 ml of acetic anhydride were added and slowly heated to 60°C and maintained at that temperature. After completion of the reaction, it was cooled for 3 hours. Further cooling to 10-30°C and stirring to induce crystallization was carried out for 2 hours, followed by diafiltration. The filter cake was washed with an appropriate amount of purified water and then dried under vacuum at 50 °C for 8 hours to give 418.3 g of a white solid, aceclofenac, in 97.1% yield and 99.80% HPLC purity. The largest single impurity was 0.04% 2-(tert-butoxy)-2-oxoethyl 2-(2-((2,6-dichlorophenyl)amino)phenyl)acetate. 418.3 g of crude aceclofenac was transferred to a 2L three-necked flask, 840 ml of acetic acid was added and heated until the solid was completely dissolved. After dissolution, 8.4 g of activated charcoal was added and stirred for 1 h. Hot filtration was performed. The filtrate was cooled to 25°C and stirred to crystallize for 4 hours, followed by filtration. The filter cake was washed with an appropriate amount of purified water to neutrality and then dried under vacuum at 50°C for 24 hours. A white solid with a dry weight of 412.0 g was obtained as a refined product of aceclofenac with a refined yield of 98.5%, an HPLC purity of 99.96%, and a maximum single impurity 2-(tert-butoxy)-2-oxoethyl 2-(2-((2,6-dichlorophenyl)amino)phenyl)acetate content of 0.005%.
Potentially hazardous interactions with other drugs
ACE inhibitors and angiotensin-II antagonists:
antagonism of hypotensive effect; increased risk of
nephrotoxicity and hyperkalaemia.
Analgesics: avoid concomitant use of 2 or more
NSAIDs, including aspirin (increased side effects);
avoid with ketorolac (increased risk of side effects
and haemorrhage).
Antibacterials: possible increased risk of convulsions
with quinolones.
Anticoagulants: effects of coumarins and
phenindione enhanced; possibly increased risk of
bleeding with heparins, dabigatran and edoxaban -
avoid long term use with edoxaban.
Antidepressants: increased risk of bleeding with
SSRIs and venlaflaxine.
Antidiabetic agents: effects of sulphonylureas enhanced.
Antiepileptics: possibly increased phenytoin
concentration.
Antivirals: increased risk of haematological toxicity
with zidovudine; concentration possibly increased by
ritonavir.
Ciclosporin: may potentiate nephrotoxicity
Cytotoxics: reduced excretion of methotrexate;
increased risk of bleeding with erlotinib.
Diuretics: increased risk of nephrotoxicity;
antagonism of diuretic effect, hyperkalaemia with
potassium-sparing diuretics.
Lithium: excretion decreased.
Pentoxifylline: increased risk of bleeding.
Tacrolimus: increased risk of nephrotoxicity.
About two-thirds of a dose is excreted in the urine,
mainly as hydroxymetabolites, the principal one being
4-hydroxyaceclofenac. A small amount is converted to
diclofenac.
[1] Patent: CN108383745, 2018, A. Location in patent: Paragraph 0029-0031; 0032-0034; 0035-0037; 0038-0069
[2] Patent: US2004/24057, 2004, A1. Location in patent: Page/Page column 53