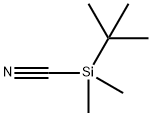
bp 163–167°C/760 mmHg; mp 76–78°C.
tert-Butyldimethylsilyl cyanide may be used as reagent for the formation of β-isonitrile alcohols via epoxides.
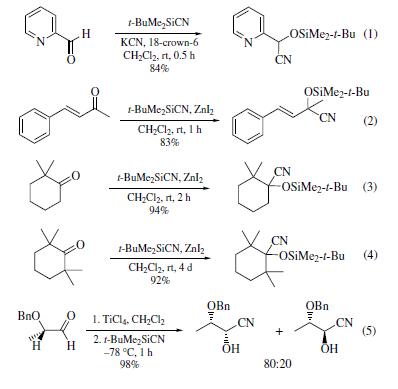
The Lewis acid-catalyzed reaction of
t-butyldimethylsilyl cyanide(TBDMSCN)with aldehydes and ketones
affords the corresponding silylated cyanohydrins in good
yield (eq 1).These have a greater stability than the silylated
cyanohydrins obtained by using cyanotrimethylsilane The addition of TBDMSCN to sterically hindered ketones proceeds
smoothly in the presence of a catalytic amount of zinc iodide or
potassium cyanide/18-crown-6 (eqs 2–4).Achelation-controlled
stereoselective synthesis of silylated cyanohydrins has also been
reported (eq 5).
conveniently prepared by refluxing
t-butyldimethylchlorosilane (1 equiv), potassium cyanide
(1.3 equiv) and 18-crown-6 (0.31 equiv) in dry methylene chloride
under nitrogen.The reagent can also be prepared by
the reaction of silver(I) cyanide with t-butyldimethylchlorosilane,
or stirring at 60°C for 4 h a mixture of sodium cyanide,
Amberlite XAD-4 resin, and t-butyldimethylchlorosilane in
acetonitrile
tert-Butyldimethylsilyl cyanide (TBDMSCN) is a bulkier trialkylsilylcyanide. It participates in the cyanosilylation of enantiopure 4-oxoazetidine-2-carbaldehydes. Addition of TBDMSCN to sterically hindered ketones in the presence of Lewis acid or base catalyst has been studied. ZnI2-catalyzed addition of TBDSCN to 2,2-dimethylcyclohexanone, 2,2,6-trimethylcyclohexanone and 2,2,6,6-tetramethylcyclohexanone affords protected cyanohydrins.