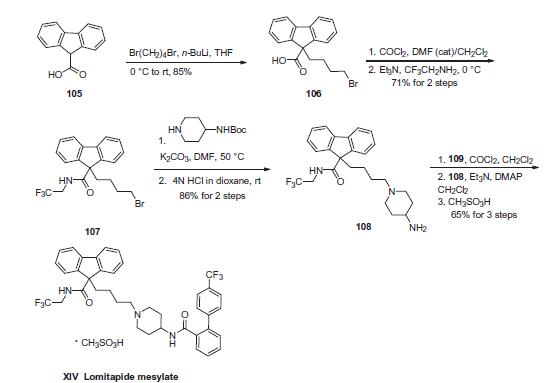
Commercial 9H-fluorene-9-carboxylic acid (105) was alkylated
with 1,4-dibromobutane in the presence of n-butyl lithium in
THF to give 9-(4-bromobutyl)-9H-fluorene-9-carboxylic acid
(106) in 85% yield. Next, activation of the acid as the acid chloride
followed by coupling with (2,2,2-trifluoroethylamine) provided
amide 107 in 71% yield for the two-step sequence. Displacement
of the terminal bromide with the appropriate 4-carbamoyl
piperidine followed by removal of the Boc group furnished piperidinyl
fluorine 108 in high yield. Amine 108 was then reacted with
the acid chloride derived from acid 109 (derived from the Suzuki
coupling of boronic acid 110 and o-iodobenzoic acid 111) to give
lomitapide, and this was followed by salt formation with methanesulfonic
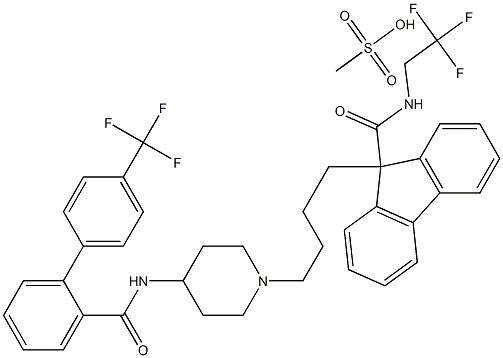
acid to afford lomitapide mesylate (XIV).