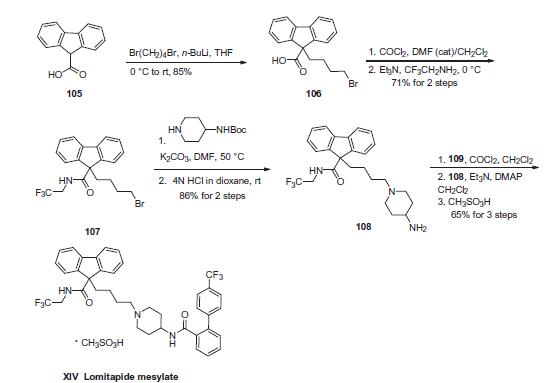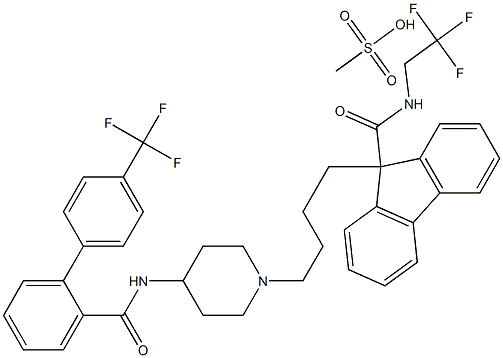
BMS 201038-04
- Product NameBMS 201038-04
- CAS202914-84-9
- CBNumberCB02627605
- MFC40H41F6N3O5S
- MW789.8260592
- MDL NumberMFCD19443682
- MOL File202914-84-9.mol
Chemical Properties
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO:100.0(Max Conc. mg/mL);126.61(Max Conc. mM) Ethanol:100.0(Max Conc. mg/mL);126.61(Max Conc. mM) |
| form | Solid |
| color | White to off-white |
| CAS DataBase Reference | 202914-84-9 |
| FDA UNII | X4S83CP54E |