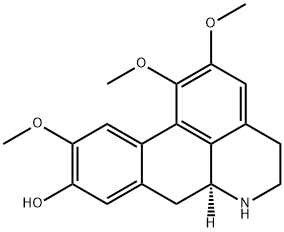
The noraporphine alkaloid was first isolated by Greshoff from Litsea chrysocorna
and subsequently from other species of Lauraceae including L. citrata and L.
cubeba. When freshly crystallized from Me2 CO it yields colourless crystals but
these become yellow in air. The alkaloid behaves as a phenolic base giving salts
with mineral acids which all contain H20 of crystallization. The picrate monohydrate has m.p. 1480 C, the dibenzoyl derivative, m.p. 169-1700 C and the
phenylthiocarbamide, m.p. 211-2°C. Three methoxyl groups, an imino group
and a phenolic hydroxyl group are present, the latter giving the O-methyl ether
which is an amorphous powder but yields a crystalline hydrochloride, m.p.
245°C; an oxalate, m.p. 233°C and the thiocarbamide, m.p. 154-5°C. The Nmethyl derivative (q.v.) is found naturally in Peurnus boldus and the O,Ndimethyl derivative is glaucine. The structure has been determined from chemical
degradation and synthesis.
Greshoff., Ber., 23,3537 (1890)
Filippo., Arch. Pharm., 236, 601 (1898)
Gorter., Bull. Jard. bot. Buitenzorg., 3, 180 (1921)
Barger, Silberschmidt.,J. Chem. Soc., 2919 (1928)
Barger et al., Ber., 66, 450 (1933)
Ruegger., Helv. Chim. Acta, 42,754 (1959)
Kikkawa.,J. Pharm. Soc., Japan, 79,83,425 (1959)