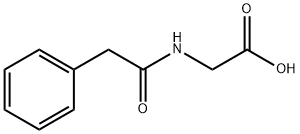
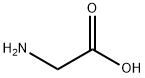
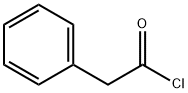
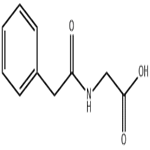
Glycine (100 mg, 1.33 mmol) was dissolved in 2 M aqueous sodium hydroxide solution (2 mL) followed by the addition of acetone (2 mL) and the reaction mixture was cooled in an ice bath. Phenylacetyl chloride (161 μL, 1.21 mmol) was slowly added dropwise while maintaining the same temperature. After the dropwise addition, the reaction system was gradually warmed up to room temperature and stirred continuously for 6 hours. Upon completion of the reaction, the solvent was removed by rotary evaporator and the residue was adjusted to pH 2-3 with 1 M hydrochloric acid solution and subsequently extracted with ethyl acetate. The organic phases were combined, dried with anhydrous magnesium sulfate, filtered and concentrated to give phenylacetylglycine (190 mg, 81% yield) as a white solid. The product was characterized by 1H NMR (300 MHz, acetone-d6): δ= 7.41-7.20 (m, 5H), 3.95 (d, J = 5.74 Hz, 1H), 3.59 (s, 2H).13C NMR (75 MHz, DMSO-d6): δ= 171.53, 170.81, 136.32, 129.25, 128.36, 126.55, 42.15, 40.95. MALDI-TOF-MS analysis: calculated value of 194.1 and measured value of 194.1 [M + H]+ for C10H12NO3.