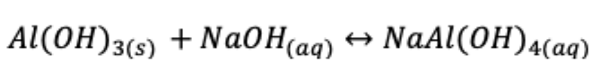Aluminium oxide is often produced by the Bayer process, which means refining bauxite to produce alumina. The following reversible chemical equation describes the grounds of the Bayer process:
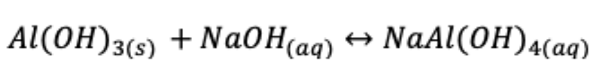
This process starts by drying crushed and washed bauxite, usually containing 30–55% Al2O3 [4]. The bauxite is dissolved in caustic soda to form a slurry, heated to temperatures of about 230–520 °F (110–270 °C). This mixture is then filtered to remove the residue called the “red mud” impurities. The filtered alumina solution (aluminum hydroxide) is then transferred or pumped into precipitator tanks, where it cools and starts to seed. These seeds stimulate a precipitation process allowing solid aluminium hydroxide crystals to be formed. All the aluminium hydroxide that settles at the bottom of the tank is removed. The remaining caustic soda is washed away from aluminium hydroxide, which undergoes various levels of filtering. Finally, it is heated to completely remove excess water. After passing through a cooling stage, the fine white powder is produced.