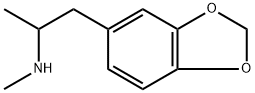
3,4-Methylenedioxymethamphetamine (MDMA) is an Entactogen; It is the N-methyl derivative of MDA. The (S)-form of MDMA possesses more potent CNS activity than the (R) form of MDMA.MDMA is controlled substance (hallucinogen).
Four biotransformation pathways of 3,4-
(methylenedioxy)methamphetamine (MDMA) in rats
are identified as N-demethylation, O-dealkylation,
deamination, and conjugation (O-methylation, O-
glucuronidation, and/or sulfation). Specific MDMA
metabolites identified are 3-hydroxy-4-
methoxymethamphetamine, 4-hydroxy-3-
methoxymethamphetamine, 3,4-
dihydroxymethamphetamine, 4-hydroxy-3-
methoxyamphetamine, 3,4-
(methylenedioxy)amphetamine (MDA), (4-hydroxy-3-
methoxyphenyl)acetone, [3,4-
(methylenedioxy)phenyl]acetone, and (3,4-
dihydroxyphenyl)acetone. MDMA is metabolized by 10 000 g rat liver supernatant to 4-hydroxy-3-
methoxymethamphetamine, 3,4-
dihydroxymethamphetamine, MDA, and [3,4-
(methylenedioxy)phenyl]acetone. Also, the 10 000 g
rat brain supernatant metabolizes MDMA to 4-hydroxy-
3-methoxymethamphetamine, 3,4-
dihydroxymethamphetamine, 4-hydroxy-3-
methoxyamphetamine, and MDA.