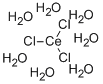colourless crystalline solid
Catalyst employed in a solvent-free microwave-assisted synthesis of 2-quinolones via condensation of ?-ketoesters with o-anilinoketones. Coupled with NaI promotes the Beckmann rearrangement of oximes.
Cerium(III) chloride heptahydrate can be used in the conversion of esters to allylsilanes.
Cerium(III) chloride heptahydrate is used in the preparation of allylsilanes from esters. It is used as a reducing agent in organic synthesis in place of sodium borohydride. In Luche reaction, carvone gives selectively allylic alcohol.
Cerium(III) chloride heptahydrate can be used:
As a precursor to prepare cerium oxide nanoparticles for biomedical applications and photocatalytic degradation.
As a solution to fabricate thin films of CeO2 on glass substrates by the spray pyrolysis process.
As a dopant to fabricate ZnO and CeO2 nanocrystals for electrochemical sensing of H2O2 and photocatalytic degradation of Rhodamine B and Congo red dyes.
As an additive to prepare corrosion-inhibiting formulations and coatings.
To synthesize carbon nanofiber composites to fabricate high-temperature polymer electrolyte membrane fuel cell cathodes.
As a support for the combination of cerium(III)chloride heptahydrate and sodium iodide supported on silica gel to promoteMichael-type additions. These catalysts are used to convert from indolesand nitroalkenes to 2-indolyl-1-nitroalkane derivatives in good yields.
Cerium(III) chloride heptahydrate is a mild Lewis acid. It participates in the allylation of aldehydes. Cerium(III) chloride heptahydrate in combination with sodium iodide supported on silica gel efficiently promotes Michael-type addition. Cerium(III) chloride heptahydrate in the presence of sodium iodide acts as an efficient catalyst for the Michael addition of 1,3-dicarbonyl compounds to α,β-unsaturated ketones and α,β-unsaturated aldehydes.
reagent type: catalyst
core: cerium