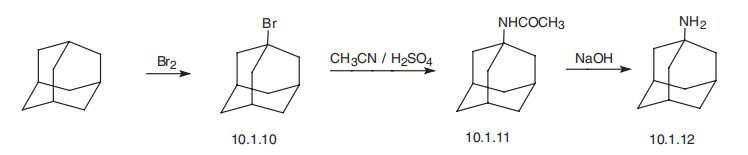
Adamantanamine
- Product NameAdamantanamine
- CAS768-94-5
- MFC10H17N
- MW151.25
- EINECS212-201-2
- MOL File768-94-5.mol
Chemical Properties
| Melting point | 206-208 °C(lit.) |
| Boiling point | 263.29°C (rough estimate) |
| Density | 0.9510 (rough estimate) |
| refractive index | 1.5220 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | 1 M HCl: soluble5%, clear to hazy, colorless to faint yellow or tan |
| form | Powder |
| pka | 10.1(at 25℃) |
| color | White to cream |
| Water Solubility | Soluble in organic solvents. Insoluble in water. |
| Merck | 14,374 |
| BRN | 2204333 |
| InChIKey | DKNWSYNQZKUICI-UHFFFAOYSA-N |
| CAS DataBase Reference | 768-94-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Tricyclo[3.3.1.13,7]decane-1-amine(768-94-5) |
| EPA Substance Registry System | Tricyclo[3.3.1.13,7]decan-1-amine (768-94-5) |
Safety Information
| Hazard Codes | Xn,Xi |
| Risk Statements | 22-36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
| RTECS | YD1925000 |
| F | 10-34 |
| HS Code | 29213000 |
| Hazardous Substances Data | 768-94-5(Hazardous Substances Data) |
| Toxicity | LD50 oral in rat: 900mg/kg |