polymer
Polymer is the product made of the polymerization reaction of monomer. Molecule should have repetitive structural units. Polymer of low molecular weight is called as low polymer (or oligomer) such as trioxymethylene. Polymer of high-molecular weight, up to thousands or even millions is called as high polymer or high-molecule compound. There are two types of high polymers including natural products and artificially synthetic product. Natural polymer such as protein is the polymers of amino acids while the starch and cellulose are the polymers (polysaccharides) of cyclic polyhydroxy, also known as biopolymers. Most of them are biodegradable and may cause short-term water BOD increment. It can also cause decomposition to generate pollutants such as ammonia, hydrogen sulfide and methane under anaerobic conditions, but will not cause long-term environmental impact. There are many types of synthetic polymers with their products having wide application. Related products include polyvinyl chloride and polystyrene plastics, resins, polyester and rubber. They are non-biodegradable substance. Long-term existence in the environment can cause a huge amount of garbage waste, among which the plasticizer will be evaporated out to pollute the environment, affecting human health at the same time. The monomers of polymer are mostly derived from petroleum, many of which are toxic and harmful substances, such as vinyl chloride which is a carcinogen. Nowadays, people are developing various polymer-containing oxide, peroxide-containing compounds and carbonyl-containing compounds. The polymer, under the sunlight photolysis, will be converted to low molecular weight organic compounds and further become harmless bio-degradable substance without contaminating the environment.
High molecular polymer is a compound consisting of one or several major structural units connecting with each other by covalent bond, also known polymers, high-molecule compound, macromolecular compounds and the like. E.g., polyethylene [-CH2-CH2-] n, Nylon 6 [-NH (CH2) 5CO-] n, whose structural units are respectively-CH2-CH2-, -NH (CH2) 5CO- with the polymerization degree being n.
Because of the high molecular weight (usually 104 to 106), it displays a number of special properties, such as relatively refractory soluble, or insoluble; swelling before being dissolved; much higher solution viscosity than a solution of equal concentration of small molecules; it has great intermolecular forces with usually exhibiting as only viscous liquid or solid form, not able to subject to gasification; the solid has a certain mechanical strength and can be subject to snagging and drawing.
The polymer is consists of a lot of macromolecular polymer chains of varying lengths, this feature is called the polydispersity of the molecular weight. We can use the distribution of the molecule weight to describe its polydispersity. The commonly referred molecular weight of the polymer is the average molecular weight of the polymer. Depending on the different statistical averaging method, there is number average molecular weight [equal to the total mass of the polymer (in g unit) divided by the total amount of the contained molecules of various molecule weights to represent Mm], weight average molecular weight (equal to the sum of the multiply of the molecules of various molecule weight and their corresponding molecular weight, expressed in Mm) and so on. Mm / Mm is called as polydispersity index, used to measure the width of the molecular weight distribution. The greater, the polydispersity index, the wider the molecular weight distribution will be and the greater the degree of dispersion.
The properties of polymers are still largely determined by the shape of the molecular chain. Depending on the shape of the molecular chain, it can be divided into linear, ball-type, network type and body-type, several structures.
The atoms of the main chain in the linear-structure polymer are often arranged in the shape of a long chain and the main chain is connected with in more or less amount of branches of varying lengths. When the polymer of such structure is heated, they tend to be melted, but also be soluble in certain organic solvents, having the probability of forming a crystal and can be oriented artificially. As some small amounts of branched-chain contained in increases the molecular spacing, the structure becomes loose, thereby reducing mechanical strength but increasing the solubility and plasticity. Common polymers belonging to this type include polyethylene, polyvinyl chloride and rubber.
The main chain of the ball-shape-structure polymer is also in long chain shape but with a large number of branches surrounding the main chain, making the molecule become globular. Its strength and elasticity is as high as that of the linear structure with no significant melting point but with excellent solubility. Phenolic resins, urea-formaldehyde resin which are the intermediate of reactions both belong to such structures.
The main chain of the high polymer of network structure is also in long chain shape, but has the bonds cross-linked, forming mesh-shape. It does not undergo melting at high temperatures but can be softened to have plasticity; it is not dissolved in an organic solvent but can swell, i.e. vulcanized rubber belongs to such type of polymer.
The high polymer of body structure has the long-chain as the main chain, being formed through the crosslink with many other molecules in three-dimensional space. However, during the process of polymerization of monomers, it can also be gradually formed through stepwise cross-linking.
The polymer of body structure is hard and brittle and can neither be melted in the high temperature nor do have plasticity. It can’t be dissolved in an organic solvent. The final products of phenol, aldehyde amine, epoxy and polyester, etc. all belong to this structure.
We can make classification for the polymer from different perspectives. According to the property and purpose, it can be divided into chemical fiber, plastics, rubber, paint and adhesives; According to the name of the synthetic reaction, it can be divided into addition polymer (such as polyethylene, polypropylene, etc.), condensation polymers (such as polyesters, polyamides etc.), ring-opening polymer (e.g., polyether, etc.); according to the source, it can be divided into natural polymers (e.g. starch, cellulose, etc.), synthetic polymers (e.g. vinyl polymers, etc.), semi-synthetic polymers (e.g. acetic acid cellulose and so on); according to the elements of the main chain, it can be divide into carbon-chain polymer (the main chain is mainly composed of carbon atoms), hetero chain polymer (the main chain contains, in addition to carbon atoms, oxygen, nitrogen, sulfur and other hetero atoms as well), element-organic polymer (main chain mainly composed of boron, silicon, aluminum, oxygen, nitrogen, sulfur and phosphorus atoms, but side-chain consists of organic groups such as methyl, ethyl, etc.); according to the application functions, it can be divided into general polymer, functional polymer and so on.
The major index of the polymers include strength, hardness, heat resistance, corrosion resistance, abrasion resistance, solvent resistance, light transmittance, and air tightness as well as electrically insulating properties. Density of the polymer is small, being much smaller than that of steel of the same volume; some of them are conductive, magnetic, and some are high temperature resistant, low temperature resistant and radiation resistant; some have excellent air tightness, transparency and so on. It has been widely used in medical, electrical conductivity, heat resistance, construction, packaging materials, plastic and some other fields.
The method of producing a polymer include bulk polymerization (including melt polymerization, referring to that monomer undergoes polymerization upon the action of light, heat and radiation without other media), solution polymerization (polymerization of monomer, initiator dissolved in an appropriate solution), emulsion (under the action of emulsifier and agitation, have the monomer be dispersed in water to form emulsion liquid to have polymerization), suspended polymerization (under the action of stirring and a dispersing agent, a monomer is dispersed into monomer droplets for being suspended in water for polymerization), etc., according to different requirements, we can apply different polymerization methods.
- Structure:
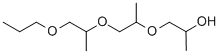
- Chemical Name:TRI(PROPYLENE GLYCOL) PROPYL ETHER
- CAS:96077-04-2
- MF:C12H26O4
- Chemical Name:Release agent No.8
- CAS:
- MF:
- Structure:
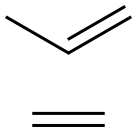
- Chemical Name:POLY(PROPYLENE-CO-ETHYLENE)
- CAS:9010-79-1
- MF:(C3H6.C2H4)x
- Chemical Name:FEMA 2501
- CAS:8023-91-4
- MF:
- Structure:
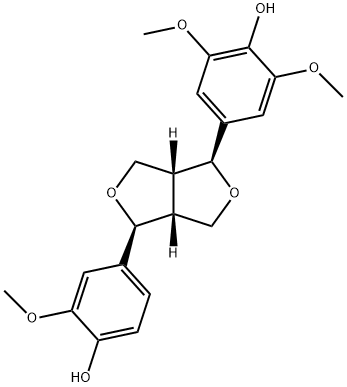
- Chemical Name:Medioresil
- CAS:40957-99-1
- MF:C21H24O7
- Chemical Name:Liquid Terpene Resin
- CAS:
- MF:
- Structure:
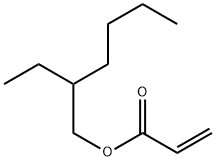
- Chemical Name:POLY(2-ETHYLHEXYL ACRYLATE)
- CAS:9003-77-4
- MF:C11H20O2
- Structure:
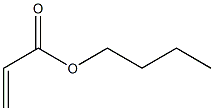
- Chemical Name:Butyl acrylate resin
- CAS:9003-49-0
- MF:C7H12O2
- Chemical Name:AMBERLITE(R) IRA-35
- CAS:76930-03-5
- MF:
- Structure:
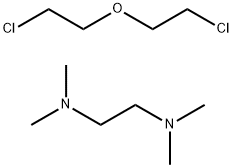
- Chemical Name:Mayosperse 60
- CAS:31075-24-8
- MF:C10H24Cl2N2O
- Chemical Name:DUOLITE C-26 ION-EXCHANGE RESIN
- CAS:68441-33-8
- MF:(C10H10.C8H8.Na)x
- Chemical Name:TRIPHENYLPHOSPHINE RESIN, 1% DVB; 100-200 MESH; 1-1.5 MMOL/G
- CAS:
- MF:
- Structure:
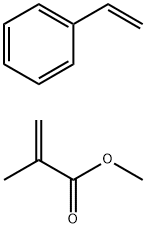
- Chemical Name:POLY(STYRENE-CO-METHYL METHACRYLATE)
- CAS:25034-86-0
- MF:C13H16O2
- Structure:
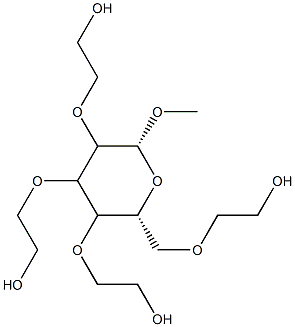
- Chemical Name:METHYL GLUCETH-20
- CAS:68239-42-9
- MF:C15H30O10
- Structure:
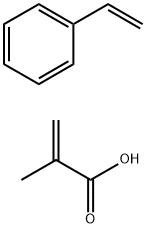
- Chemical Name:SODIUM STYRENE/ACRYLATES COPOLYMER
- CAS:9010-92-8
- MF:C12H14O2
- Structure:
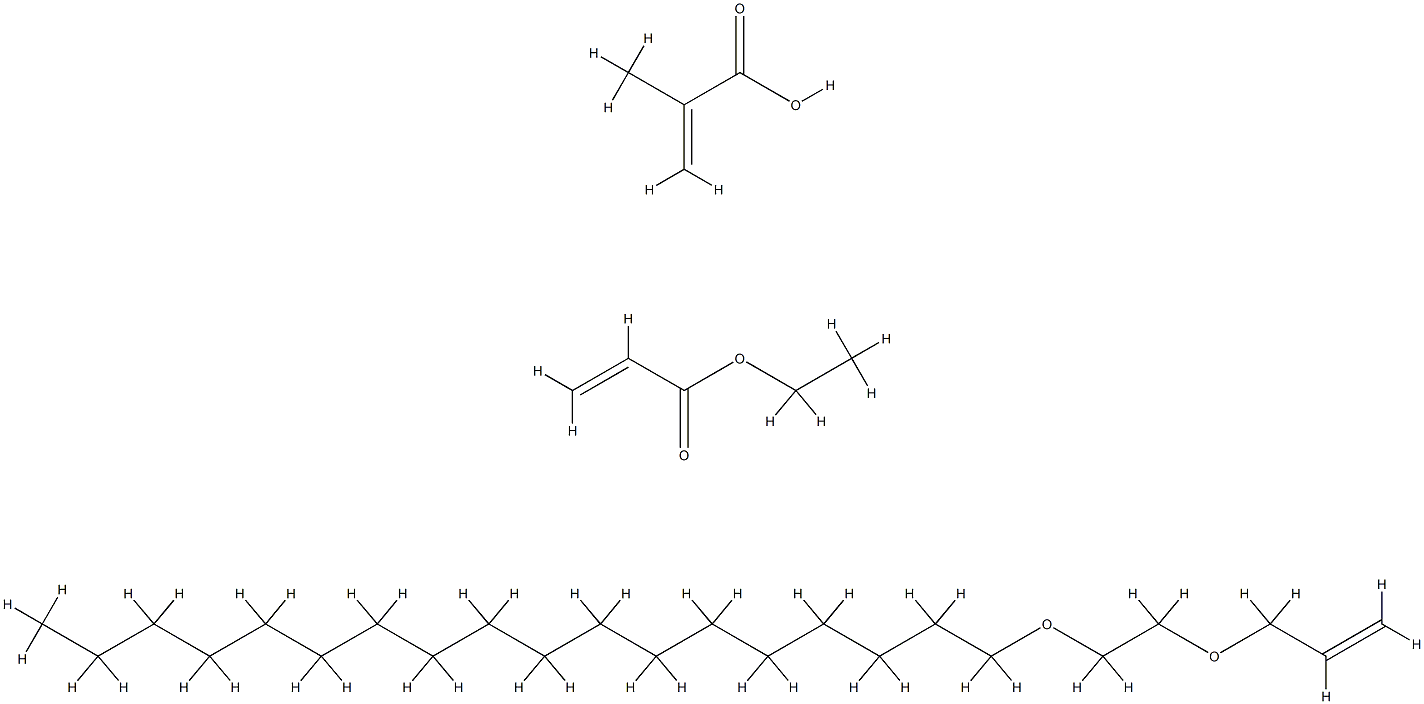
- Chemical Name:STEARETH-10 ALLYL ETHER/ACRYLATES COPOLYMER
- CAS:109292-17-3
- MF:C32H60O6
- Chemical Name:POLYGLYCERYL-10 DIISOSTEARATE
- CAS:63705-03-3
- MF:C39H80O7
- Structure:
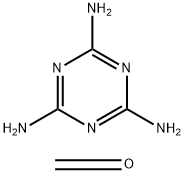
- Chemical Name:POLY(MELAMINE-CO-FORMALDEHYDE), BUTYLATED
- CAS:68002-25-5
- MF:C4H8N6O
- Structure:
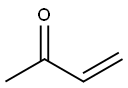
- Chemical Name:POLY(VINYL METHYL KETONE)
- CAS:25038-87-3
- MF:C4H6O
- Structure:
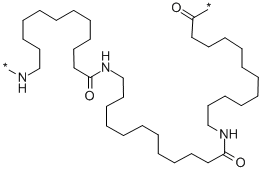
- Chemical Name:NYLON 12
- CAS:25038-74-8
- MF:C36H69N3O3X2
- Structure:
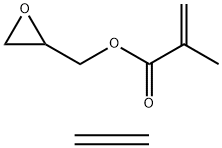
- Chemical Name:POLY(ETHYLENE-CO-GLYCIDYL METHACRYLATE)
- CAS:26061-90-5
- MF:C9H14O3
- Structure:
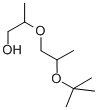
- Chemical Name:DI(PROPYLENE GLYCOL) TERT-BUTYL ETHER
- CAS:132739-31-2
- MF:C10H22O3
- Chemical Name:INDOLE RESIN, 1% DVB; 0.7-1.4 MMOL/G; 100-200 MESH
- CAS:
- MF:
- Structure:
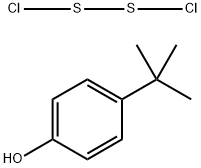
- Chemical Name:Poly-tert-butylphenoldisulfide
- CAS:60303-68-6
- MF:C10H14Cl2OS2
- Structure:
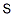
- Chemical Name:Insoluble sulfur
- CAS:9035-99-8
- MF:S
- Structure:
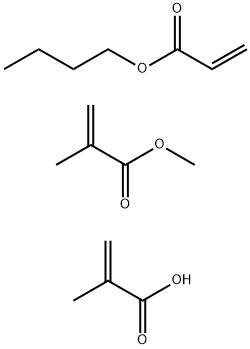
- Chemical Name:Methacrylic acid, polymer with butyl acrylate and methyl methacrylate
- CAS:25035-69-2
- MF:C16H26O6
- Structure:
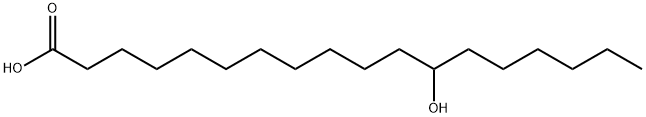
- Chemical Name:POLYHYDROXYSTEARIC ACID
- CAS:27924-99-8
- MF:C18H36O3
- Structure:
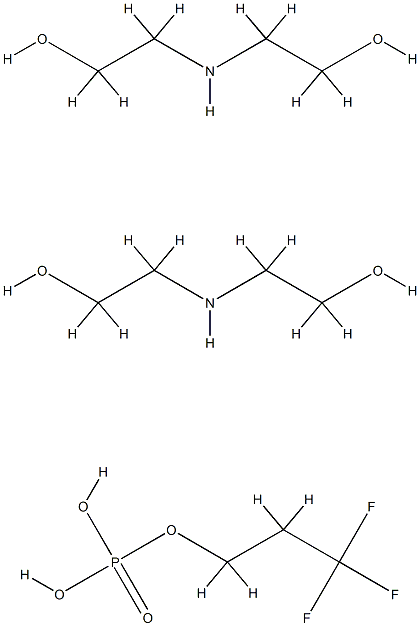
- Chemical Name:DEA-C8-18 PERFLUOROALKYLETHYL PHOSPHATE
- CAS:65530-63-4
- MF:C11H28F3N2O8P
- Chemical Name:Polyacrylic resin Ⅱ
- CAS:
- MF:
- Chemical Name:Merrifield resin
- CAS:
- MF:
- Chemical Name:Chelating resin
- CAS:
- MF:
- Structure:
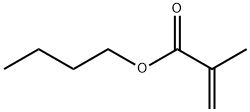
- Chemical Name:POLY(N-BUTYL METHACRYLATE)
- CAS:9003-63-8
- MF:C8H14O2
- Chemical Name:POLY(1-DECENE)
- CAS:68649-12-7
- MF:[CH2CH[(CH2)7CH3]]n
- Chemical Name:AMBERLITE IRA-404
- CAS:149146-26-9
- MF:
- Structure:
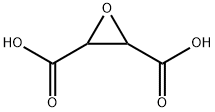
- Chemical Name:Polyepoxysuccinic Acid
- CAS:51274-37-4
- MF:C4H4O5
- Structure:
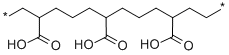
- Chemical Name:POLY(ETHYLENE-CO-ACRYLIC ACID)
- CAS:9010-77-9
- MF:(C3H4O2.C2H4)x
- Structure:
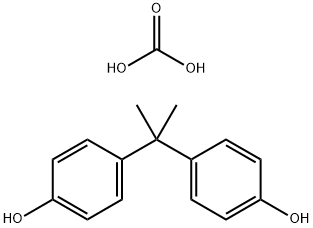
- Chemical Name:Polycarbonate
- CAS:25037-45-0
- MF:C16H18O5
- Structure:
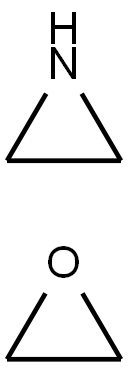
- Chemical Name:POLYETHYLENEIMINE
- CAS:26658-46-8
- MF:C4H9NO
- Structure:
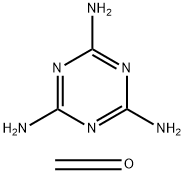
- Chemical Name:MELAMINE RESIN
- CAS:9003-08-1
- MF:(C3H6N6.CH2O)x
- Structure:
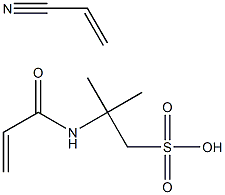
- Chemical Name:POLY(2-ACRYLAMIDO-2-METHYL-1-PROPANESULFONIC ACID-CO-ACRYLONITRILE)
- CAS:54640-82-3
- MF:C10H16N2O4S
- Structure:
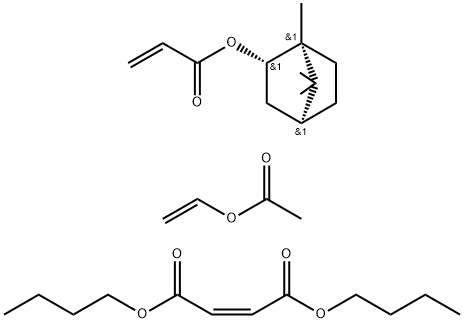
- Chemical Name:POLY(VINYL ACETATE-CO-BUTYL MALEATE-CO-ISOBORNYL ACRYLATE)
- CAS:136392-68-2
- MF:C29H46O8
- Structure:
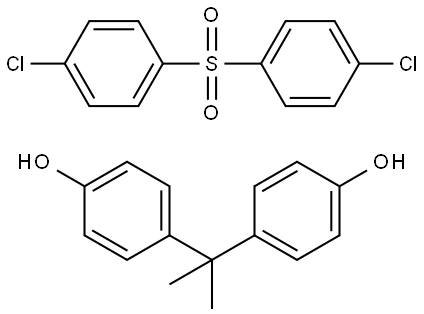
- Chemical Name:POLYSULFONE
- CAS:25154-01-2
- MF:(C15H16O2.C12H8Cl2O2S)x
- Structure:
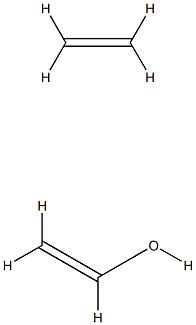
- Chemical Name:Poly(vinyl alcohol-co-ethylene)
- CAS:25067-34-9
- MF:C4H8O
- Structure:
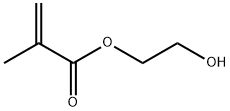
- Chemical Name:POLY(2-HYDROXYETHYL METHACRYLATE)
- CAS:25249-16-5
- MF:C6H10O3
- Structure:
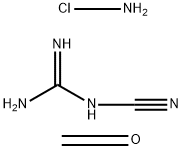
- Chemical Name:POLY(METHYLENE-CO-GUANIDINE), HYDROCHLORIDE
- CAS:55295-98-2
- MF:C3H8ClN5O
- Chemical Name:POLYETHYLENE, CHLORINATED
- CAS:64754-90-1
- MF:
- Structure:
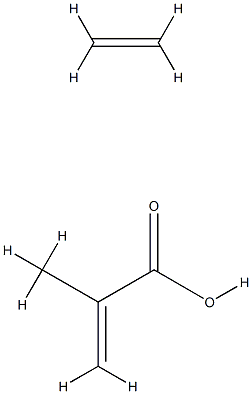
- Chemical Name:POLY(ETHYLENE-CO-METHACRYLIC ACID)
- CAS:25053-53-6
- MF:C6H10O2
- Structure:
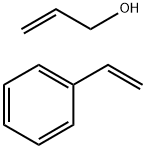
- Chemical Name:STYRENE/ALLYL ALCOHOL COPOLYMER
- CAS:25119-62-4
- MF:C11H14O
- Structure:
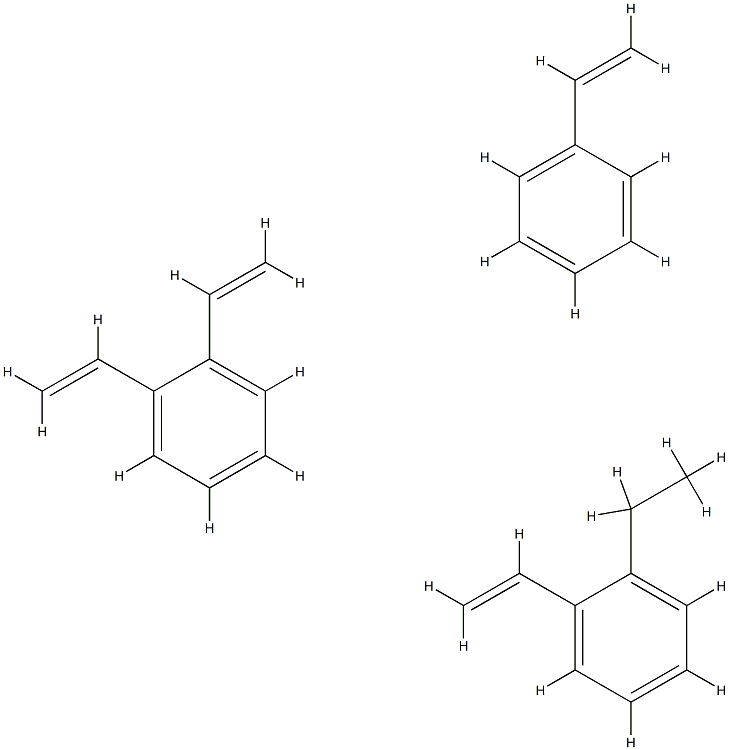
- Chemical Name:PORAPAK P (100-120 MESH ASTM) FOR GC
- CAS:9052-95-3
- MF:C28H30
- Chemical Name:Petroleum resins
- CAS:64742-16-1
- MF:
- Structure:
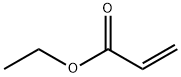
- Chemical Name:POLY(ETHYL ACRYLATE)
- CAS:9003-32-1
- MF:C5H8O2
- Structure:
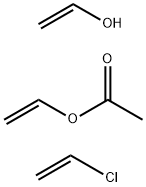
- Chemical Name:POLY(VINYL CHLORIDE-CO-VINYL ACETATE-CO-VINYL ALCOHOL)
- CAS:25086-48-0
- MF:C8H13ClO3
- Structure:
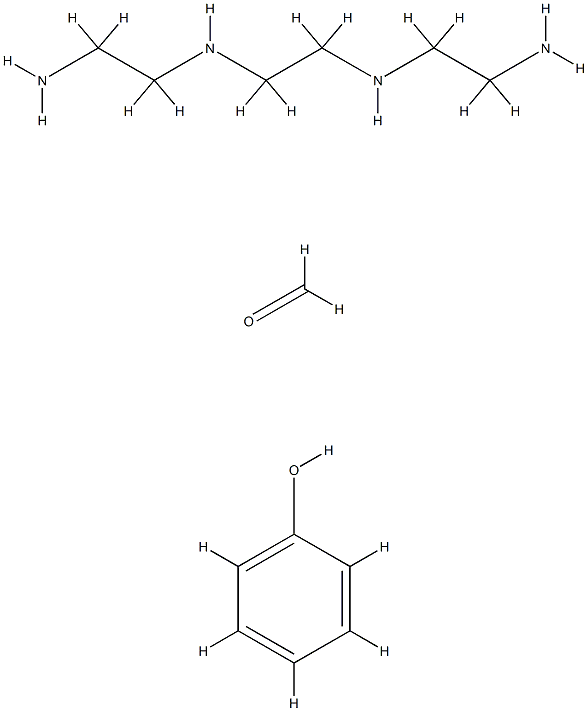
- Chemical Name:DUOLITE A-7 ION-EXCHANGE RESIN
- CAS:32610-77-8
- MF:C13H26N4O2
- Structure:
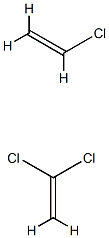
- Chemical Name:POLY(VINYLIDENE CHLORIDE-CO-VINYL CHLORIDE)
- CAS:9011-06-7
- MF:C4H5Cl3
- Structure:
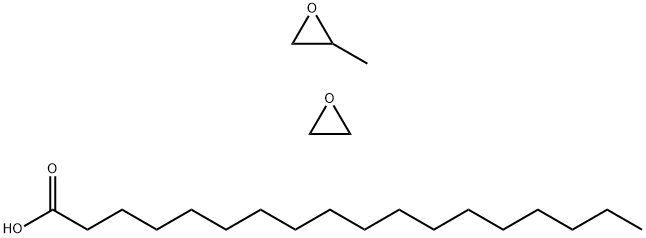
- Chemical Name:POLYOXYETHYLENE 25 PROPYLENE GLYCOL STEARATE
- CAS:37231-60-0
- MF:C23H46O4
- Structure:
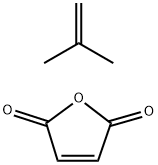
- Chemical Name:POLY(ISOBUTYLENE-CO-MALEIC ACID) SODIU&
- CAS:39612-00-5
- MF:C8H10O3
- Structure:
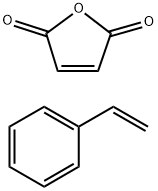
- Chemical Name:POLY(STYRENE-ALT-MALEIC ACID) SODIUM
- CAS:25736-61-2
- MF:C12H10O3
- Structure:
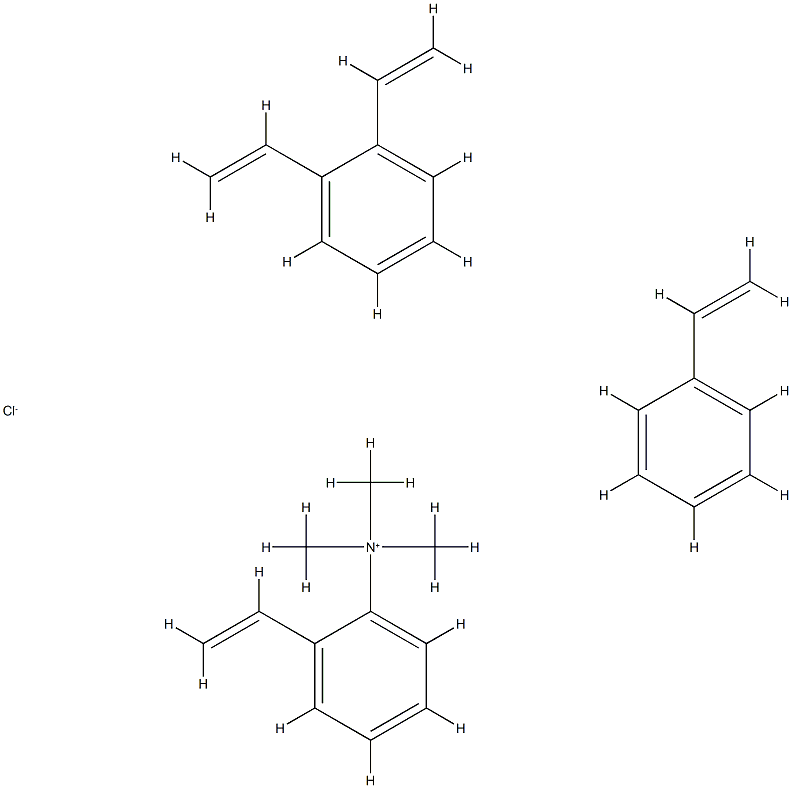
- Chemical Name:Anion exchang resin
- CAS:122560-63-8
- MF:C29H34ClN
- Chemical Name:Polypropylene polyol diphenylmethanediisocyanate prepolymer
- CAS:9048-57-1
- MF:C15H10N2O2.H.(C3H6O)n.OH
- Structure:
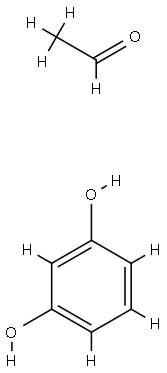
- Chemical Name:Poly(acetaldehyde-resorcinol)
- CAS:28410-56-2
- MF:C8H10O3
- Structure:
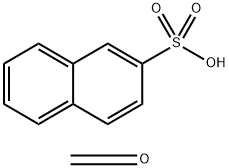
- Chemical Name:Sodium salt of polynaphthalene sulphonic acid
- CAS:36290-04-7
- MF:C11H10O4S
- Chemical Name:Diclofenac resinate
- CAS:
- MF:
- Structure:
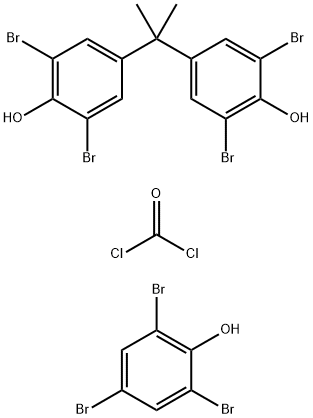
- Chemical Name:TBBPA carbonate oligomer BC58
- CAS:71342-77-3
- MF:C22H15Br7Cl2O4
- Structure:
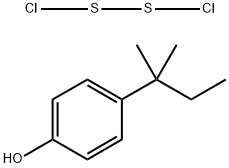
- Chemical Name:Phenol, 4-(1,1-dimethylpropyl)-, polymer with sulfur chloride (S2Cl2)
- CAS:68555-98-6
- MF:C11H16Cl2OS2
- Chemical Name:Amberlite LA-2
- CAS:11128-96-4
- MF:
- Structure:
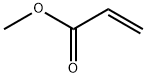
- Chemical Name:POLY(METHYL ACRYLATE)
- CAS:9003-21-8
- MF:C4H6O2
- Chemical Name:TRIMETHYLOLPROPANE TRIS[POLY(PROPYLENE GLYCOL), AMINE TERMINATED] ETHER
- CAS:39423-51-3
- MF:C2H5C[CH2[OCH2CH(CH3)]nNH2]3
- Structure:
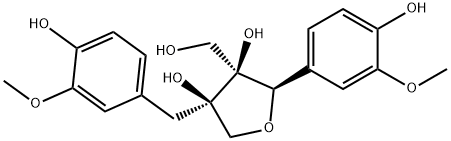
- Chemical Name:Massoniresil
- CAS:96087-10-4
- MF:C20H24O8
- Structure:
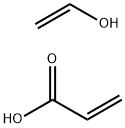
- Chemical Name:POLY(ACRYLIC ACID), SODIUM SALT-GRAFT-POLY(ETHYLENE OXIDE)
- CAS:27599-56-0
- MF:C5H8O3
- Chemical Name:IMINODIACETIC ACID RESIN
- CAS:11139-85-8
- MF:NULL
- Structure:
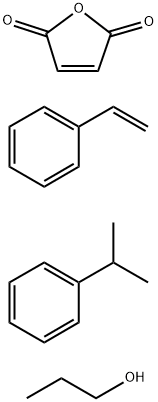
- Chemical Name:POLY(STYRENE-CO-MALEIC ACID), PARTIAL PROPYL ESTER, CUMENE TERMINATED
- CAS:160611-48-3
- MF:C24H30O4
- Chemical Name:HMQ silicone resin
- CAS:
- MF:
- Structure:
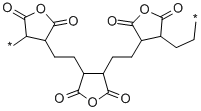
- Chemical Name:POLY(ETHYLENE-ALT-MALEIC ANHYDRIDE)
- CAS:9006-26-2
- MF:(C4H2O3.C2H4)x
- Chemical Name:POLY(PROPYLENE GLYCOL) (300) MONOMETHACRYLATE
- CAS:39420-45-6
- MF:(C3H6O)nC4H6O2
- Structure:
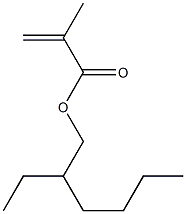
- Chemical Name:POLY(2-ETHYLHEXYL METHACRYLATE)
- CAS:25719-51-1
- MF:C12H22O2
- Structure:
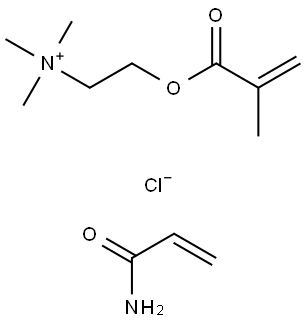
- Chemical Name:Polyquaternium-32
- CAS:35429-19-7
- MF:(C9H18NO2.C3H5NO.Cl)x
- Structure:
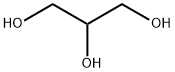
- Chemical Name:Polyglycerine
- CAS:25618-55-7
- MF:C3H8O3
- Structure:
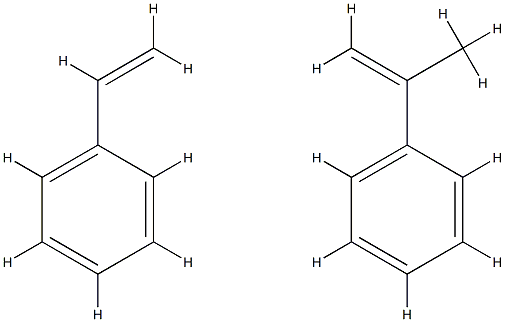
- Chemical Name:POLY(STYRENE-CO-ALPHA-METHYLSTYRENE)
- CAS:9011-11-4
- MF:(C9H10.C8H8)x
- Structure:
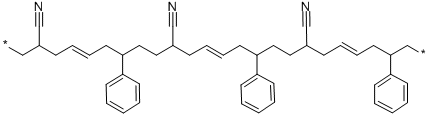
- Chemical Name:ABS Resins
- CAS:9003-56-9
- MF:C45H51N3X2
- Structure:
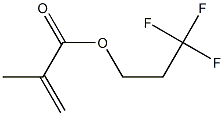
- Chemical Name:2-(Perfluoroalkyl)ethyl methacrylate
- CAS:65530-66-7
- MF:C7H9F3O2
- Structure:
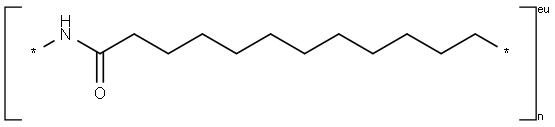
- Chemical Name:Nylon 12
- CAS:24937-16-4
- MF:(C12H23NO)n
- Chemical Name:AMBERLITE DP-1
- CAS:64333-21-7
- MF:
- Chemical Name:CHELATING RESIN (IMINODIACETIC ACID)
- CAS:
- MF:
- Structure:
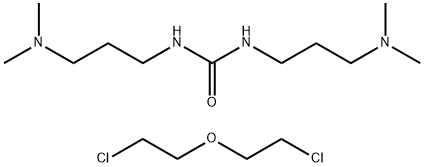
- Chemical Name:Polyquaternium-2
- CAS:68555-36-2
- MF:C15H34Cl2N4O2
- Structure:
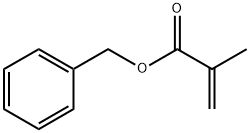
- Chemical Name:POLY(BENZYL METHACRYLATE)
- CAS:25085-83-0
- MF:C11H12O2
- Structure:
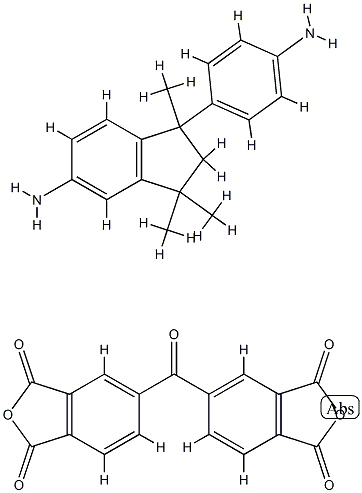
- Chemical Name:POLYIMIDE RESIN
- CAS:62929-02-6
- MF:C35H28N2O7
- Structure:
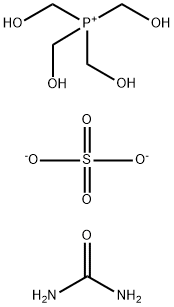
- Chemical Name:Tetrakis(hydroxymethyl)phosphonium sulfate urea polymer
- CAS:63502-25-0
- MF:C5H16N2O9PS-
- Chemical Name:PPG-12/SMDI COPOLYMER
- CAS:9042-82-4
- MF:(C15H22N2O2.(C3H6O)nH2O)x
- Structure:

- Chemical Name:Polylactic acid
- CAS:26023-30-3
- MF:(C3H4O2)n
- Chemical Name:inorganic resin
- CAS:
- MF:
- Chemical Name:Methyl MQ resin
- CAS:
- MF:
- Structure:
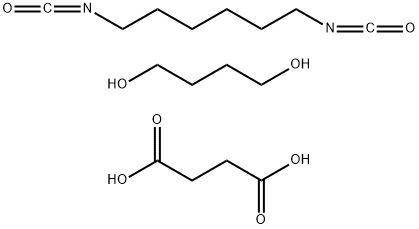
- Chemical Name:POLY(1,4-BUTYLENE SUCCINATE), EXTENDED WITH 1,6-DIISOCYANATOHEXANE
- CAS:143606-53-5
- MF:C16H28N2O8
- Chemical Name:Carfopol resin
- CAS:
- MF:
- Structure:
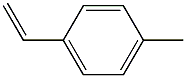
- Chemical Name:POLY(4-METHYL STYRENE)
- CAS:24936-41-2
- MF:C9H10
- Chemical Name:(PHENYLSILSESQUIOXANE)-(DIMETHYLSILOXANE) COPOLYMER
- CAS:73138-88-2
- MF:
- Structure:
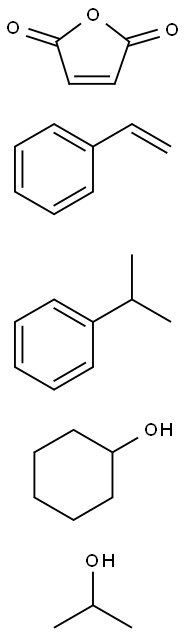
- Chemical Name:POLY(STYRENE-CO-MALEIC ACID), PARTIAL CYCLOHEXYL/ISOPROPYL ESTER, CUMENE TERMINATED
- CAS:160611-51-8
- MF:C30H42O5
- Structure:
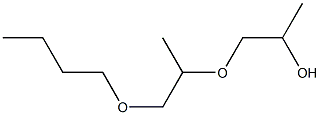
- Chemical Name:ANTIFOAM PE-L
- CAS:9003-13-8
- MF:C10H22O3
- Structure:
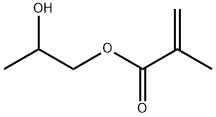
- Chemical Name:POLY(2-HYDROXYPROPYL METHACRYLATE)
- CAS:25703-79-1
- MF:C7H12O3
- Structure:
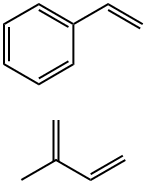
- Chemical Name:BUTYLENE/ETHYLENE/STYRENE COPOLYMER
- CAS:68648-89-5
- MF:C13H16
- Structure:
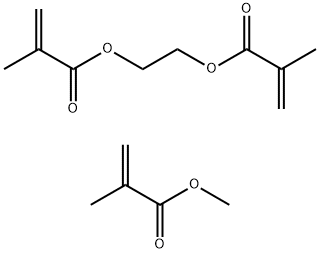
- Chemical Name:METHYL METHACRYLATE CROSSPOLYMER
- CAS:25777-71-3
- MF:C15H22O6