Alkylating antineoplastic
In all kinds of anti-tumor chemotherapies, Alkylating agent is the earliest and most widely used application of the anticancer drugs, and it is also one of the biggest families of anti-tumor chemotherapies. Since the application of nitrogen mustard treatment of malignant tumors from 1942, alkylating agents has become one of the most important type of chemotherapeutic drugs.
The main features of these drugs is a common molecular structure cytotoxic components that molecules containing alkyl group usually contains one or two alkyl groups, and therefore are called as single-function or bifunctional alkylating agent. These alkyl groups typically can be converted to the electron-deficient active intermediate products, which could covalent bind with the electron-donating group (eg an amino group, a mercapto group, a hydroxyl group, a carboxylic acid group, a phosphoric acid group, etc. ) contained in the biomacromolecules of cells (such as DNA, RNA and protein) and then there occurs alkylation reaction. This could cause the loss of these cellular components in cell metabolism, composition of mutated cells and cell division, resulting in cell death. Compared with other anticancer drugs, this type of drugs has little resistance, and there is little cross resistance reaction between alkylating agents and non-alkylating agents as well as between alkylating agents. Even if there is reaction, it’s a mild reaction. Bone marrow suppressions and gastrointestinal reactions are common untoward effects of this type of drugs. The most commonly used alkylating antineoplastic agents can be divided into these several types: mustard and its derivatives (diclofenac diethylamine class), ethylene imines, methane sulfonic acid esters, nitrosourea, epoxides and others.
- Structure:
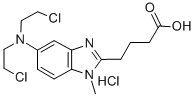
- Chemical Name:Bendamustine hydrochloride
- CAS:3543-75-7
- MF:C16H22Cl3N3O2
- Structure:
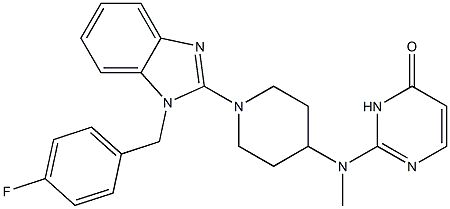
- Chemical Name:Mizolastine
- CAS:108612-45-9
- MF:C24H25FN6O
- Structure:
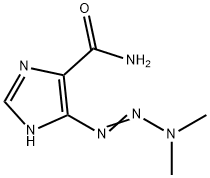
- Chemical Name:Dacarbazine
- CAS:4342-03-4
- MF:C6H10N6O
- Structure:
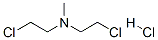
- Chemical Name:Mechlorethamine hydrochloride
- CAS:55-86-7
- MF:C5H12Cl3N
- Structure:
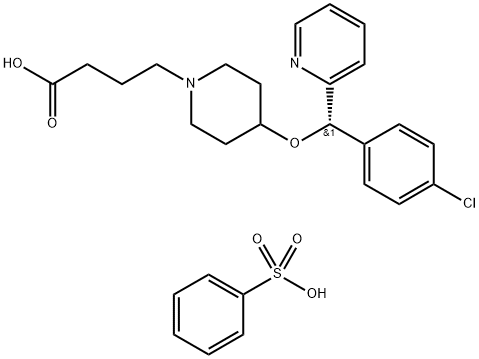
- Chemical Name:BEPOTASTINE BESILATE
- CAS:190786-44-8
- MF:C27H31ClN2O6S
- Structure:
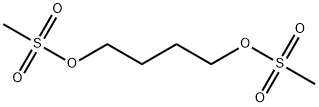
- Chemical Name:Busulfan
- CAS:55-98-1
- MF:C6H14O6S2
- Structure:
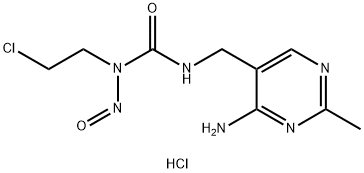
- Chemical Name:Nimustine hydrochloride
- CAS:55661-38-6
- MF:C9H14Cl2N6O2
- Structure:
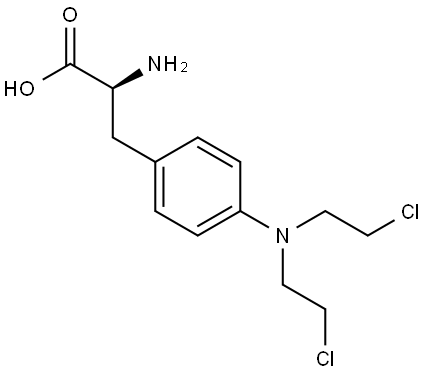
- Chemical Name:Melphalan
- CAS:148-82-3
- MF:C13H18Cl2N2O2
- Structure:
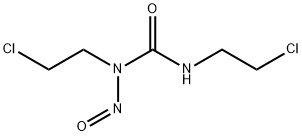
- Chemical Name:Carmustine
- CAS:154-93-8
- MF:C5H9Cl2N3O2
- Structure:
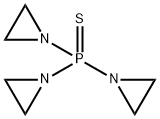
- Chemical Name:Triethylenethiophosphoramide
- CAS:52-24-4
- MF:C6H12N3PS
- Structure:
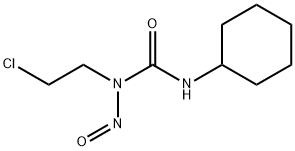
- Chemical Name:Lomustine
- CAS:13010-47-4
- MF:C9H16ClN3O2
- Structure:
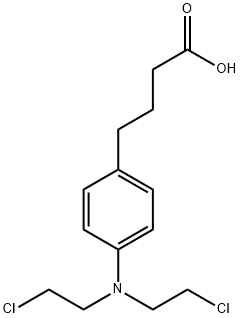
- Chemical Name:Chlorambucil
- CAS:305-03-3
- MF:C14H19Cl2NO2
- Structure:
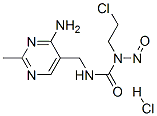
- Chemical Name:NIMUSTINE HYDROCHLORIDE
- CAS:52208-23-8
- MF:C9H14Cl2N6O2
- Structure:
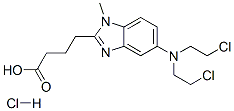
- Chemical Name:Bendamustine
- CAS:97832-05-8
- MF:C16H21Cl2N3O2.HCl
- Structure:
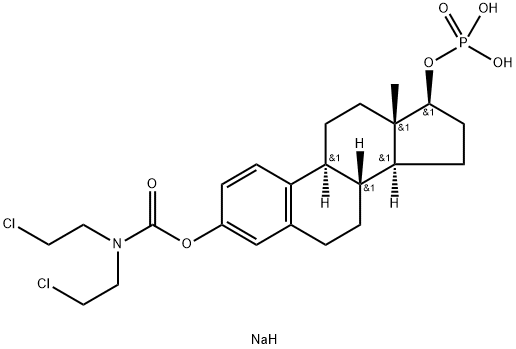
- Chemical Name:Estramustine sodium phosphate
- CAS:52205-73-9
- MF:C23H33Cl2NNaO6P
- Structure:
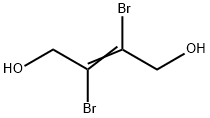
- Chemical Name:trans-2,3-Dibromo-2-butene-1,4-diol
- CAS:3234-02-4
- MF:C4H6Br2O2
- Structure:
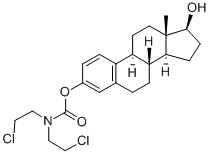
- Chemical Name:Estramustine
- CAS:2998-57-4
- MF:C23H31Cl2NO3
- Structure:
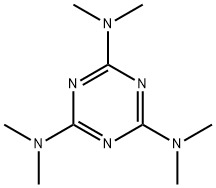
- Chemical Name:Altretamine
- CAS:645-05-6
- MF:C9H18N6
- Structure:
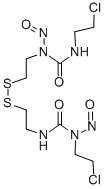
- Chemical Name:Ditiomustine
- CAS:82599-22-2
- MF:C10H18Cl2N6O4S2
- Structure:
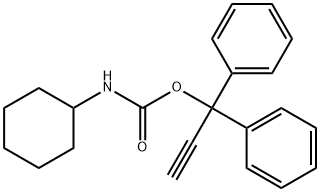
- Chemical Name:Enpromate
- CAS:10087-89-5
- MF:C22H23NO2
- Structure:
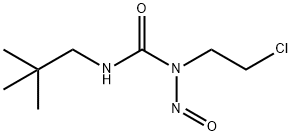
- Chemical Name:Neptamustine
- CAS:73105-03-0
- MF:C8H16ClN3O2
- Chemical Name:GLYFOSFIN
- CAS:
- MF:
- Structure:

- Chemical Name:NITROCAPHANE
- CAS:
- MF:C14H19Cl2N3O4
- Structure:
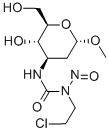
- Chemical Name:Ecomustine
- CAS:98383-18-7
- MF:C10H18ClN3O6
- Chemical Name:Methoxymerphalan
- CAS:
- MF:
- Structure:
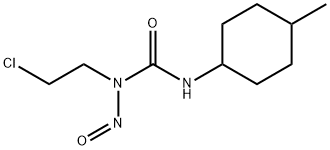
- Chemical Name:Semustine
- CAS:13909-09-6
- MF:C10H18ClN3O2
- Structure:
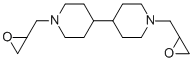
- Chemical Name:epipropidine
- CAS:5696-17-3
- MF:C16H28N2O2
- Structure:
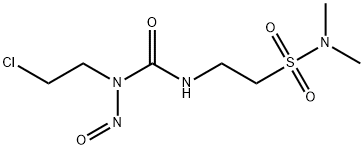
- Chemical Name:Tauromustine
- CAS:85977-49-7
- MF:C7H15ClN4O4S
- Structure:
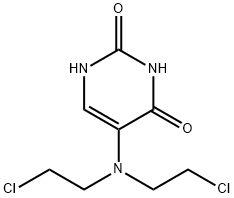
- Chemical Name:URACIL MUSTARD
- CAS:66-75-1
- MF:C8H11Cl2N3O2
- Structure:
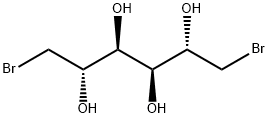
- Chemical Name:1,6-DIBROMO-1,6-DIDEOXY-D-MANNITOL
- CAS:488-41-5
- MF:C6H12Br2O4
- Structure:
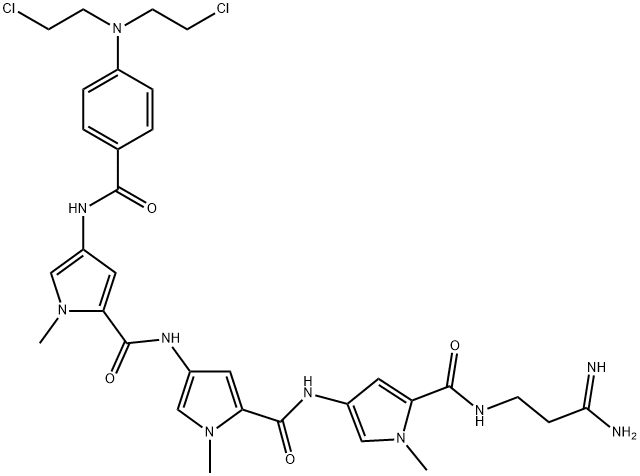
- Chemical Name:Tallimustine
- CAS:115308-98-0
- MF:C32H38Cl2N10O4
- Structure:
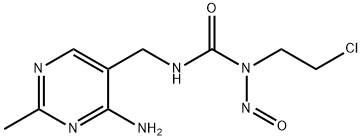
- Chemical Name:Nimustine
- CAS:42471-28-3
- MF:C9H13ClN6O2
- Chemical Name:BendaMustine IMpurity B
- CAS:
- MF:
- Structure:
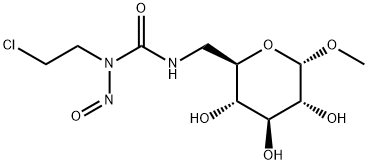
- Chemical Name:Ranimustine
- CAS:58994-96-0
- MF:C10H18ClN3O7
- Structure:
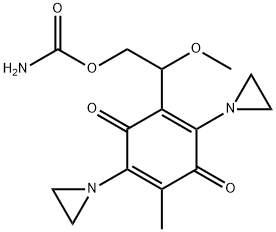
- Chemical Name:carboquone
- CAS:24279-91-2
- MF:C15H19N3O5
- Structure:
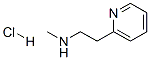
- Chemical Name:Betahistine Hydrochloride
- CAS:15430-48-5
- MF:C8H13ClN2
- Structure:
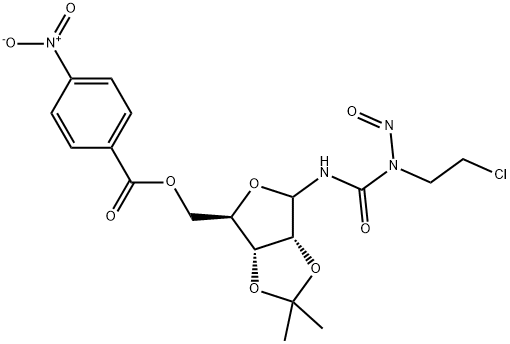
- Chemical Name:Bofumustine
- CAS:55102-44-8
- MF:C18H21ClN4O9
- Structure:
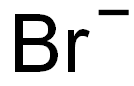
- Chemical Name:BendaMustine IMpurity A
- CAS:109882-26-0
- MF:Br-
- Structure:
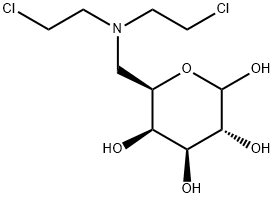
- Chemical Name:Galamustine
- CAS:105618-02-8
- MF:C10H19Cl2NO5
- Structure:
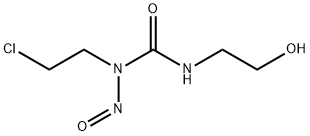
- Chemical Name:elmustine
- CAS:60784-46-5
- MF:C5H10ClN3O3
- Structure:
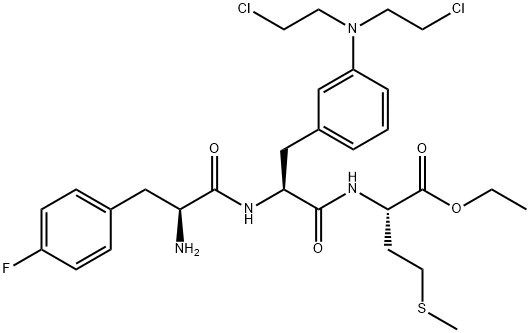
- Chemical Name:Ambamustine
- CAS:85754-59-2
- MF:C29H39Cl2FN4O4S
- Structure:
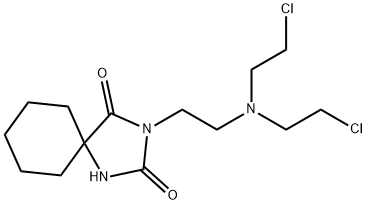
- Chemical Name:Spiromustine
- CAS:56605-16-4
- MF:C14H23Cl2N3O2
- Structure:
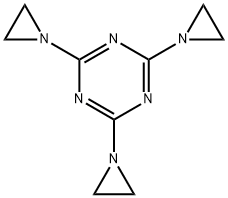
- Chemical Name:TRIETHYLENEMELAMINE
- CAS:51-18-3
- MF:C9H12N6
- Structure:
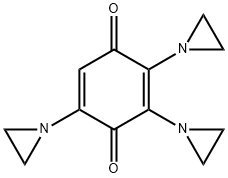
- Chemical Name:trisethyleneiminoquinone
- CAS:68-76-8
- MF:C12H13N3O2
- Structure:
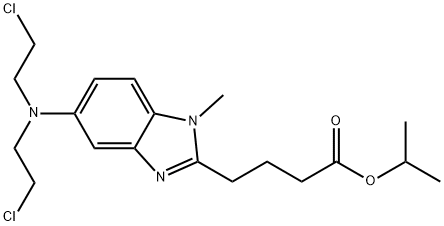
- Chemical Name:BendaMustine IMpurity C
- CAS:1313020-25-5
- MF:C19H27Cl2N3O2
- Structure:
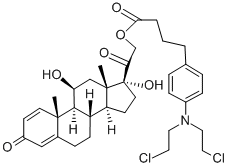
- Chemical Name:prednimustine
- CAS:29069-24-7
- MF:C35H45Cl2NO6
- Chemical Name:Formylmerphalan
- CAS:
- MF:
- Chemical Name:Predmustine
- CAS:
- MF:
- Chemical Name:BETAMERPHALAN
- CAS:
- MF:
- Structure:
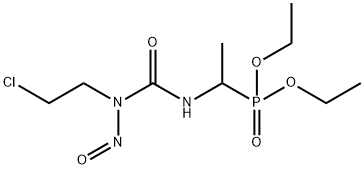
- Chemical Name:Fotemustine
- CAS:92118-27-9
- MF:C9H19ClN3O5P
- Structure:
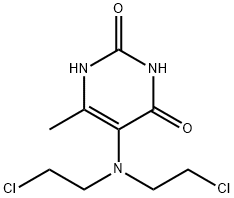
- Chemical Name:2,6-DIHYDROXY-4-METHYL-5-[BIS(2-CHLOROETHYL)AMINO]PYRIMIDINE
- CAS:520-09-2
- MF:C9H13Cl2N3O2
- Structure:
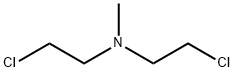
- Chemical Name:Chlormethine
- CAS:51-75-2
- MF:C5H11Cl2N
- Structure:
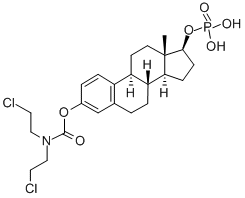
- Chemical Name:estra-1,3,5(10)-triene-3,17beta-diol 3-[bis(2-chloroethyl)carbamate] 17-(dihydrogen phosphate)
- CAS:4891-15-0
- MF:C23H32Cl2NO6P
- Chemical Name:Estramustine Na PH
- CAS:
- MF:
- Chemical Name:Dianhydrodulcitol
- CAS:
- MF:
- Chemical Name:Solaziquone
- CAS:
- MF:
- Chemical Name:OCAPHANE
- CAS:
- MF:
- Chemical Name:Mechlorethaminoxide
- CAS:
- MF:
- Structure:
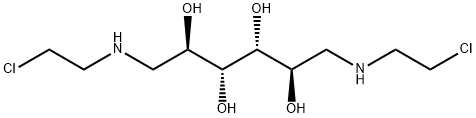
- Chemical Name:mannomustine
- CAS:576-68-1
- MF:C10H22Cl2N2O4