Metal oxide
Metal oxides, especially metal oxides having semiconductor properties are the effective catalysts of oxidation - reduction reaction. Industrial catalysts typically contain one or more metal oxide component, called as a composite metal oxide catalyst. Generally speaking, there is at least one transition metal oxide contained it with each component forming mixture with each other at the molecular level and have interaction with each other, adjusting the electrical properties and surface acidity of the catalyst, improving the catalytic activity and selectivity.、
There are two situations regarding to the decomposition products of the metal oxide:
1. Decomposition into elemental metals and oxygen; the general principle of such reaction is that, more active the metal is , the more stable formed metal oxide will be, the more difficult formed metal oxide can be subject to decomposition; otherwise easily prone to be subject to decomposition. Only inert metal oxide can be subject to thermal decomposition; for example, mercuric oxide, silver oxide, etc; there is also some kinds of metal oxide can be subject to powered decomposition when being in the molten state.
2. High-valence metal oxide will be decomposed into low-valence oxide and oxygen when being heated metal oxide.
- Chemical Name:PLATINUM(Ⅳ) OXIDE
- CAS:
- MF:PtO2·xH2O
- Structure:

- Chemical Name:MAGNESIUM OXIDE SUBSTRATE, 10X10X0.5MM, POLISHED ONE SIDE, 111 ORIENTATION
- CAS:
- MF:MgO
- Structure:
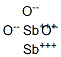
- Chemical Name:Antimony(III) Oxide
- CAS:1327-33-9
- MF:O3Sb2
- Structure:
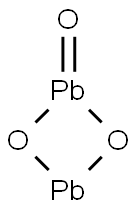
- Chemical Name:LEAD SESQUIOXIDE
- CAS:1314-27-8
- MF:O3Pb2