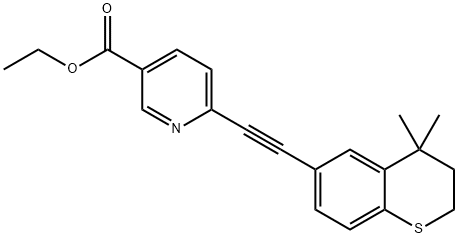
Tazarotene belongs to a third generation prescription topical retinoid. It is marketed in different forms including cream (brand name: Avage), foam (brand name: Fabuir) and gel (brand name: Tazorac). It is mainly used for the treatment of psoriasis, acne, and sun damaged skin. The detailed mechanisms of action of Tazarotene are still unclear. It is converted to its active form, the cognate carboxylic acid of tazarotene (AGN 190299) in vivo through rapid de-esterification in animals and man. AGN 190299 is capable of binding to all the three members of the retinoic acid receptor (RAR) family: RARα, RARβ, and RARγ with relative selectivity for RARβ, and RARγ. This process may further modify gene expression. However, it is unclear whether this is related to its mode of action. Common side effects associated with it include worsening of acne, desquamation, burning/stinging, erythema and pruritus increased sensitivity to sunlight, dry skin, itchiness, redness, skin pain rash and skin discoloration. In most cases, these side effects are mild and can remarkable decrease after the first 2–4 weeks. Tazarotene should not be used in the following cases: patients are allergic to it; patients are pregnant; patients have a sunburn; patients are taking photo-sensitive drugs such as thiazides and tetracycline.