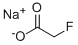
SODIUM FLUOROACETATE
$50
- Product nameSODIUM FLUOROACETATE
- Cas No62-74-8
- MFC2H2FNaO2
- MW100.02
- EINECS200-548-2
- MDL NumberMFCD00002682
Synonyms
Aceticacid,fluoro-,sodiumsalt ;compd1080 ;compoundno.1080 ;compoundno1080 ;fluoracetatedesodium ;fluorakil3 ;fluoressigaeure ;fluoressigsaeure
Properties
| Melting point | 200-205 °C (dec.) |
| Boiling point | 105-106 °C |
| vapor pressure | Non-volatile |
| solubility | DMSO (Sparingly), Methanol (Slightly) |
| form | Fine white powder |
| pka | 2.66 |
| Water Solubility | Very soluble |
| Merck | 13,4194 |
| Stability | Stable. Flammable. Risk of explosion above flashpoint. |
| CAS DataBase Reference | 62-74-8(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium fluoroacetate (62-74-8) |
Safety Information
Symbol(GHS)


Signal word
Danger
Hazard statements
H300:Fatal if swallowed
H310:Fatal in contact with skin
H330:Fatal if inhaled
H400:Very toxic to aquatic life
Precautionary statements
P260:Do not breathe dust/fume/gas/mist/vapours/spray.
P262:Do not get in eyes, on skin, or on clothing.
P264:Wash hands thoroughly after handling.
P264:Wash skin thouroughly after handling.
P270:Do not eat, drink or smoke when using this product.
P271:Use only outdoors or in a well-ventilated area.
P273:Avoid release to the environment.
P280:Wear protective gloves/protective clothing/eye protection/face protection.
P284:Wear respiratory protection.
P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician.
P302+P350:IF ON SKIN: Gently wash with plenty of soap and water.
P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing.
P310:Immediately call a POISON CENTER or doctor/physician.
P320:Specific treatment is urgent (see … on this label).
P321:Specific treatment (see … on this label).
P322:Specific measures (see …on this label).
P330:Rinse mouth.
P361:Remove/Take off immediately all contaminated clothing.
P363:Wash contaminated clothing before reuse.
P391:Collect spillage. Hazardous to the aquatic environment
P403+P233:Store in a well-ventilated place. Keep container tightly closed.
P405:Store locked up.
P501:Dispose of contents/container to..…
Description
Sodium fluoroacetate is the salt of a naturally occurring toxin
which is found in Australia, Brazil, and Africa. Naturally
occurring fluoroacetate can be found in Gastrolobium minus
(family: Fabaceae), a flowering plant in Western Australia and
often referred to as the ‘poison pea.’ Descriptions of the fluoroacetate
activity may have been described as early at 1904 in
Sierra Leone, when colonists used extracts of Chailletia toxicaria
to poison rats. The actions of fluoroacetate have also been
described in animals having ingested the poison leaf Gifblaar
(Dichapetalum cymosum). Sodium fluoroacetate was then
developed as a rodenticide and predacide in 1942 in the United
States and went under the synonym of 1080, which is its
catalog number. In the late 1970s, the use of sodium fluoroacetate
was significantly restricted in the United States due to
its high acute toxicity and the need for specialized training for
application. Additional restrictions were also imposed as to the
locations that the agent could be used and under very defined
conditions. A ‘toxic collar’ was developed which contains a very
small amount of sodium fluoroacetate (0.3 mg per collar). This
collar could be placed around the throats of livestock and
would contain chemical pouches that would be ruptured when
the animal was attacked by a predator thus restricting the
poison only to the predator. The toxicity in certain predators
(dogs, wolves, coyotes) is up to 20-fold higher than in humans.
Amphibians and other reptiles have been shown to be relatively
resistant to sodium fluoroacetate and can feed on insects
and other animals which have high levels of sodium fluoroacetate
present with no ill-effects. In the late 1980s, all use of
sodium fluoroacetate as a rodenticide was discontinued. The
availability of products containing sodium fluoroacetate is
permitted by the Environmental Protection Agency (EPA) and
the regulatory conclusion of the EPA is that these products will
not pose unreasonable risks or adverse effects if the products
are used following the restriction on the product labeling.