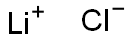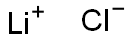Hazard statements
H302:Harmful if swallowed
H315:Causes skin irritation
H319:Causes serious eye irritation
Precautionary statements
P264:Wash hands thoroughly after handling.
P264:Wash skin thouroughly after handling.
P270:Do not eat, drink or smoke when using this product.
P280:Wear protective gloves/protective clothing/eye protection/face protection.
P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell.
P302+P352:IF ON SKIN: wash with plenty of soap and water.
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing.