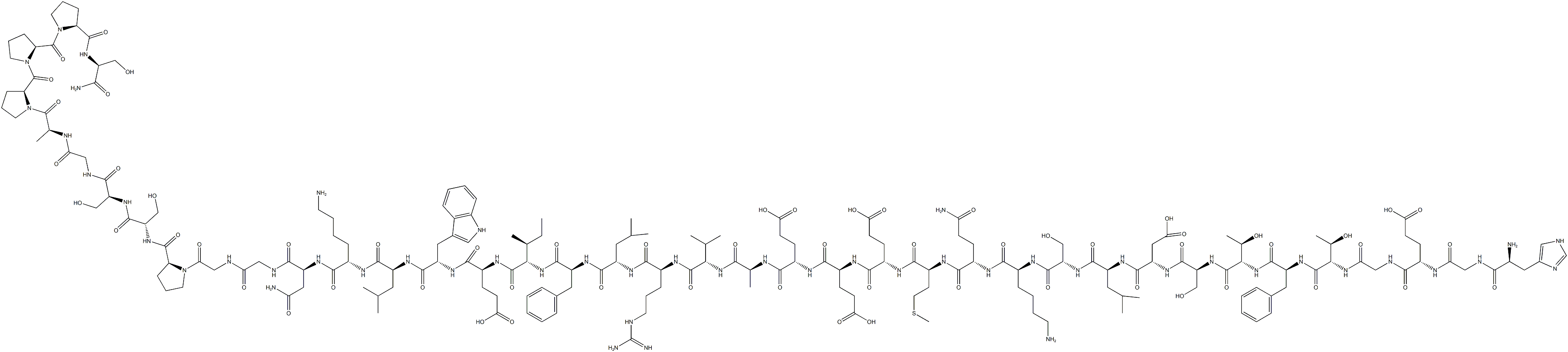
Exenatide is the first drug in a new class of anti-diabetics known as the incretin
mimetics, and it is indicated as adjunctive therapy to improve glycemic control in
patients with type 2 diabetes who are taking metformin, a sulfonylurea, or both, but
have not achieved adequate glycemic control. Exenatide is a functional analog of the
human incretin Glucagon-Like Peptide-1 (GLP-1). GLP-1 is naturally released from
cells in the GI tract in response to food intake and acts on its receptor on b-cells to
potentiate glucose-stimulated insulin secretion. Exenatide is a long-acting agonist at
the GLP-1 receptor. It is a synthetic version of a 39-amino acid peptide found in the
salivary secretions of the Gila monster lizard.also moderates peak serum glucagon levels during hyperglycemic periods following
meals, but does not interfere with glucagon release in response to hypoglycemia. The
dosing regimen for exenatide is 5 or 10 mg twice daily, administered as a subcutaneous
injection within an hour before morning and evening meals. Following subcutaneous
administration, peak plasma concentrations of exenatide are reached in
2.1 h, and the plasma pharmacokinetic profile is dose proportional. The most common adverse
events reported with exenatide include nausea, vomiting, diarrhea, feeling jittery,
dizziness, headache, and dyspepsia.